Abhijit S. Nair*, Rahul Amte*, Lavanya Ram*, T. Dasaradha Rama Reddy**
*Consultant Anesthesiologist; **Chief Orthopedic Surgeon
Yashoda Superspeciality Hospital, Somajiguda, Hyderabad (India).
Correspondence: Dr. Abhijit S. Nair, Department of Anesthesiology, Yashoda Superspeciality Hospital, Somajiguda, Hyderabad- 500084, Andhra Pradesh (India); E-mail: abhijitnair95@gmail.com
ABSTRACT
Fat embolism syndrome is commonly encountered after long bone and hip fractures.
Postoperatively it may be encountered after intramedullary nailing of long bones and pelvic
arthroplasties. We report a case involving a 17 year old girl who had both tibial bone fractures and underwent intramedullary nailing with vascular repair and developed fat embolism with lung infiltrates requiring non-invasive ventilation. We also review relevant literature and describe criteria required to diagnose fat embolism syndrome.
Key words: Fat embolism; Intramedullary nailing; Noninvasive ventilation
Citation: Nair AS, Amte R, Ram L, Reddy DR. Fat embolism syndrome due to bilateral intramedullary nailing of tibia. Anaesth Pain & Intensive Care 2014;18(3):277-279.
INTRODUCTION
Fat embolism syndrome is a condition requiring high index of suspicion. Prognosis is usually good if the condition is diagnosed early and supportive measures are started on time. It can be fatal if valuable time is lost in making a diagnosis and supportive measures are not initiated in time like oxygen supplementation, invasive or non-invasive ventilation and source control. We present a case report of a young patient who developed fat embolism after orthpedic surgery and was successfully managed with non-invasive ventilation.
CASE REPORT
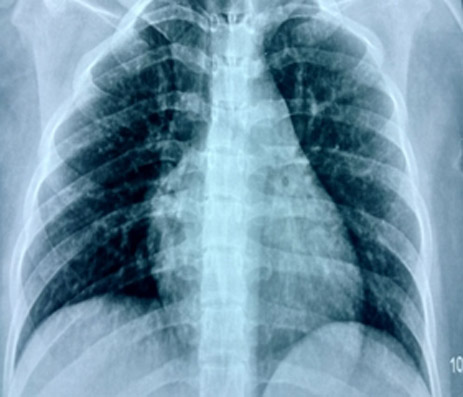
A 17 year old girl had a fall from the roof of 2nd floor and sustained fractures of both of her tibias. There was also a vascular injury to her left leg as confirmed on CT angiogram. Repair of the damaged vessel and external fixation or intramedullary (IM) nailing was planned. Her investigations were normal and she was hemodynamically stable with SPO2 of 98% on room air. Her chest radiograph was unremarkable (Figure 1).
Figure 1: Preoperative chest radiograph.
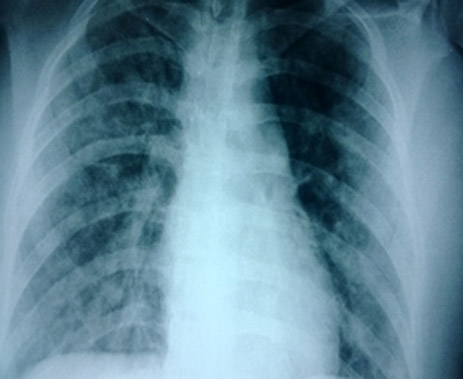
Figure 2: Immediate post operative chest radiograph showing bilateral lung infiltrates.
We administered combined spinal-epidural anesthesia at L3-4 space level under strict aseptic precautions. 3.2 ml of 0.5% heavy Bupivacaine was injected intrathecally and epidural catheter was tunnelled subcutaneously. First, the vascular surgeon repaired the damaged left anterior tibial artery and performed an embolectomy. The vascularity was confirmed with good Doppler signals over left dorsalis pedis and posterior tibial artery. Later on, the orthopedic surgeon did IM nailing for left tibia uneventfully. While doing IM nailing for the right tibial bone, the patient started coughing vigorously during reaming. Her pulse oxygen saturation dropped to 82% on room air. Oxygen supplementation was given with a face mask @ 5L of oxygen.
Saturation improved to 98% with it. However, throughout the surgery she had persistent cough. All 3 surgical procedures were finished in 2½ hours, with a blood loss of about 800 ml. Patient was shifted to SICU following surgery for further management. A postop chest radiograph was done which revealed bilateral infiltrates (Figure 2).
On blood gas analysis, PaO2 was 52 mmHg on 5 L/min of oxygen via face mask, the P:F ratio was about 125. On further evaluation, urine for fat globules was negative and 2D Echo was normal with good LV and RV function. D-dimer was negative for pulmonary thromboembolism. A diagnosis of fat embolism syndrome (FES) was considered and intermittent BIPAP with iPAP of 12 cmH2O and ePAP of 6 cmH2O was started along with levosalbutamol nebulisation 8 hourly, with incentive spirometry for 10-15 minutes 2 hourly. Injection enoxaparine 40 mg was administered subcutaneously from 1st postoperative day once daily for DVT prophylaxis. Daily chest radiograph was done to look for radiological changes. BIPAP was stopped from 3rd postoperative day, she was shifted to her room on 4th postop day and discharged from the hospital on 8th day.
DISCUSSION
FES is usually encountered after fractures of femur, tibia, pelvis and during arthroplasty of these bones. It may also be seen after massive soft tissue injury, severe burns, bone marrow biopsy, bone marrow transplant, cardiopulmonary resuscitation, liposuction and median sternotomy. It is even seen in a few non-traumatic conditions e.g. acute pancreatitis, fatty liver, corticosteroid therapy, lymphography, fat emulsion infusion and hemoglobinopathies.1 The explanations provided for manifestations of FES are presence of pulmonary AV malformations, passage of fat globules through pulmonary circulation, presence of patent foramen ovale, which leads to neurological symptoms and opening of closed foramen ovale due to increased right heart pressures due to shower of fat globules in the systemic circulation.2
Gupta et al studied the clinical profile of fat embolism syndrome in trauma patients for a period of 2 ½ years at AIIMS Apex Trauma centre, New Delhi (India), and observed that the syndrome may present in unusual forms and needs a high index of suspicion. The classical triad of fat embolism was described by Bergmann in 1873 which involves respiratory distress, altered mentation and petechial lesions 24-48 hrs after long bone fractures.3 Koul et al prospectively studied 35 patients over 3 years with suspected fat embolism syndrome and stressed on high index of suspicion after long bone fractures.4
FES occurs due to mechanical or biochemical cause. In mechanical cause, the fat globules get deposited in pulmonary circulation leading to intrapulmonary shunts which manifests as hypoxemia. In biochemical cause, the free fatty acids released by the fat globules lead to a systemic inflammatory response. The severity is more in patients with systemic comorbidities, old age and associated chest trauma. The prominent clinical features are respiratory failure requiring supplemental oxygen noninvasive or invasive ventilation, cerebral dysfunction and petechial haemorrhages on skin. Pulmonary dysfunction is the earliest to manifest and is the most common problem seen in around 75% of patients.5
Gurd’s and Wilson’s criteria is used to diagnose fat embolism syndrome which should fulfil one major and four minor criteria (Table 1). Schonfeld et al described a quantitative measure to diagnose FES; a score of more than 5 is required to diagnose FES (Table 2).
Table 1: Gurd & Wilson’s criteria
|
Major criteria |
Minor criteria |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 2: Schonfeld’s criteria
|
criteria |
Score |
| Petechiae |
5 |
| Infiltrates on x-ray |
4 |
| Hypoxemia |
3 |
| Fever |
1 |
| Tachycardia |
1 |
| Tachypnea |
1 |
| Confusion |
1 |
Lindeque et al suggested that FES can be diagnosed on the basis of respiratory system involvement alone using the criteria in Table 3.6
Table 3:Lindeque’s criteria
| Sustained pO2 < 8 kpa |
| Sustained pCO2 > 7.3 kpa |
| Sustained respiratory rate > 35/min, inspite of sedation |
| Increased work of breathing, dyspnea, tachycardia, anxiety |
A blood gas analysis is required to quantify hypoxemia. Chest radiograph helps in understanding the severity of lung injury. Serial radiographs will help in knowing the improvement. A 2D echo can help in determining cardiac cause of hypoxemia and by giving information about right heart status and pulmonary artery pressures. In established FES, there is acute pulmonary hypertension due to heavy showers of fat globules. Urine, sputum and blood can be analysed for presence of fat globules but it is not a sensitive test. In our patient blood was positive but urine was negative for fat globules. Bronchoalveolar lavage followed by staining of alveolar macrophages for fat can help in demonstrating the presence of fat globules but is not required in a patient who already has a lung injury.7,8
Fat embolism is a diagnosis of exclusion and requires a high index of suspicion. Once the diagnosis is established, the treatment is supportive. Patient requires oxygen supplementation to maintain acceptable oxygen saturation due to intrapulmonary shunt. Deep breathing exercises and incentive spirometry is very important and helpful. Role of steroids is not proven. Bederman et al did a meta analysis of 104 studies involving 389 patients where steroids were used in patients with long bone fractures, to prevent fat embolism.9 The authors suggested that a large confirmatory trial is required to prove the efficacy of steroids as the quality of trials used in the meta analysis was poor. Sen et al were of the same opinion after doing a review of seven articles involving 483 patients who received prophylactic methyl prednisolone sodium succinate for prophylaxis of fat embolism.10 They suggested large scale, uniformly designed, multi-centered, randomized, prospective trials to determine ideal situations and dosage of corticosteroids in prophylaxis of FES.
Nebulised β2 agonists and mucolytics may help. In absence of cerebral dysfunction, noninvasive ventilation (NIV) offers great help by reducing work of breathing, improves oxygenation and facilitates early recovery from acute lung injury. In presence of significant respiratory distress and severe hypoxemia, NIV should be initiated early. In severe ARDS and in presence of cerebral dysfunction, invasive ventilation is essential. Institutional protocol for deep vein thrombosis prophylaxis, nutrition and relevant antibiotic coverage should be initiated.11
Early fixation of long bone and pelvic fractures decreases the severity of fat embolism. In presence of respiratory involvement in the form of hypoxemia with or without respiratory distress, external fixation should be done because intramedullary nailing and reaming leads to recurrent showers of emboli.
CONCLUSION
The diagnosis of fat embolism requires a high index of suspicion. Supplemental oxygen and initiation of noninvasive ventilation shouldn’t be delayed once this possibility comes into mind. The prognosis is usually good if diagnosed early and supportive measures are started in time.
REFERENCES
- Shaikh N. Emergency management of fat embolism syndrome. J Emerg Trauma Shock 2009;2:29-33. [PubMed][Free Full Text]
- George J, George R, Dixit R, Gupta RC, Gupta N. Fat embolism syndrome. Lung India 2013;30:47–53. [PubMed][Free Full Text]
- Gupta B, D’souza N, Sawhney C, Farooque K, Kumar A, Agrawal P et al. Analyzing fat embolism syndrome in trauma patients at AIIMS Apex Trauma Center, New Delhi, India. J Emerg Trauma Shock 2011;4:337-41. [PubMed][Free Full Text]
- Koul PA, Ahmad F, Gurcoo SA, Khan UH, Naqash IA, Sidiq S, et al. Fat embolism syndrome in long bone trauma following vehicular accidents: Experience from a tertiary care hospital in north India. Lung India 2013;30:97-102. [PubMed][Free Full Text]
- Chhabra B, Kiran S, Senthilnathan TA, Gupta R. Fat embolism syndrome. Ind J Orthop 2001;35:10-15.
- Kwiatt ME, Seamon MJ. Fat embolism syndrome. Int J Crit Illn Inj Sci 2013;3:64–68. [PubMed][Free Full Text]
- Bhalla T, Sawardekar A, Klingele K, Tobias JD. Postoperative hypoxemia due to fat embolism. Saudi J Anaesth 2011;5:332–334. [PubMed][Free Full Text]
- Shaikh N, Parchani A, Bhat V, Kattren MA. Fat embolism syndrome: clinical and imaging considerations: case report and review of literature. Indian J Crit Care Med 2008;12:32-6. [PubMed][Free Full Text]
- Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with longbone fractures? A meta-analysis. Can J Surg 2009;52:386–393. [PubMed][Free Full Text]
- Sen RK, Tripathy SK, Krishnan V. Role of corticosteroid as a prophylactic measure in fat embolism syndrome: a literature review. Musculoskelet Surg 2012;96:1-8. [PubMed]
- Dhar D. Fat embolism syndrome. J Coll Physicians Surg Pak 2012;22:800-2. [PubMed]




 Facebook
Facebook Twitter
Twitter GooglePlus
GooglePlus Youtube
Youtube