Chakravarty Nupur, MD*, Singhai Shubha, DA, DNB**, Shidhaye Ramchandra Vinayak, MD, DA***
*Associate Professor; **Senior Resident; ***Professor
Department of Anesthesiology and Critical Care
L.N.Medical College,Bhopal,M.P. 462042 INDIA
Correspondence: Dr. Nupur Chakravarty, MD, Associate Professor of Anesthesiology, L.N. Medical College & Research Centre, J.K. Town, Sarvadharam C-Sector, Kolar Road,, Bhopal, Madhya Pradesh 462042, (India); Cell: +91 9425011099; E-mail: drnupurc@yahoo.com
ABSTRACT
Background: This study was conducted to evaluate the quality and duration of postoperative analgesia with intraperitoneal instillation of 0.5% bupivacaine after laparoscopic cholecystectomy.
Methodology: In a prospective, randomized, double blind, placebo controlled study, 60 ASA grade I and II female patients of age groups 20-60 yrs, undergoing elective laparoscopic cholecystectomy under general anesthesia were equally distributed into two groups. In Group B (study group) 20 ml of 0.5% bupivacaine and in Group S (placebo group) 20 ml of normal saline were injected intraperitoneally after gall bladder extraction. A visual analogue scale which consisted of a 10 cm scale with markings at equal intervals, where 0 represented no pain and 10 represented worst imaginable pain, was used to assess postoperative pain at predetermined time intervals. Two way repeated ANOVA test was used for studying inter group variation in different parameters over time. Pearson chi square test and unpaired t-test was applied to analyze differences in categorical and numerical variables respectively. A p value <0.05 was considered significant.
Results: The mean VAS score was less in Group B compared to Group S at all time intervals. VAS score showed greater decline between 1st and 2nd hour in Group B as compared to Group S. No difference was observed in total intra operative dose (in µg) of fentanyl (127.5 ± 12.0 vs. 126.7 ± 13.0, p>0.05) and frequency of postoperative analgesic use (3.97 ± 0.85 vs. 3.93 ± 0.83, p>0.05) in Group S vs. Group B respectively.
Conclusion: Intraperitoneal bupivacaine provides a simple technique to be used as a part of multimodal approach even though exclusive use of intraperitoneal bupivacaine as a mode of pain relief is not adequate. Use of the correct volume, dose and concentration of drug to bring about this result is of essence.
Key words: Bupivacaine, intraperitoneal; surgery, Laparoscopic cholecystectomy;
Postoperative analgesia
Citation: Chakravarty N, Singhai S, Shidhaye RV. Evaluation of intraperitoneal bupivacaine for postoperative analgesia in patients undergoing laparoscopic cholecystectomy: a prospective randomized trial. Anaesth Pain & intensive Care 2014;18(4):361-66
INTRODUCTION
The observed benefits of laparoscopic cholecystectomy, e.g. better pulmonary function, reduced post operative pain and rapid convalescence in comparison to conventional laparotomy,1,2 have made it an established surgical technique for treatment for patients with gall stone disease. However, it too, is associated with intraabdominal, incisional and shoulder tip pain after surgery, especially during first 24 post operative hours3 leading to an unwanted prolonged convalescence.4 Optimal pain management during early postoperative period is an essential component of early recovery and discharge. The complex etiology of pain after laparoscopic cholecystectomy suggests that analgesia plan has to be multimodal.5 Opioids provide good post-operative analgesia, but delay recovery and discharge from hospital.6 Non-steroidal anti inflammatory drugs (NSAID’s) have a morphine-sparing effect, but they do not appear to provide sufficient and reliable analgesia when used alone.7 Different approaches to provide additional analgesia individually, or in various combinations e.g. local anesthetic infiltration of the trocar insertion sites, low dose continuous infusion of epidural anesthetic and intraperitoneal instillation of local anesthetics have been tried.8 Clinical trials carried out to assess analgesic effect of intraperitoneal instillation of bupivacaine in varying concentrations and volumes9-12 report differing conclusions. The proposed rationale for this mechanism of analgesia is conduction block of visceral nociceptive stimuli which irritate the peritoneum, as well as absorption of drug from the large peritoneal surface.13 We conducted this study to evaluate the quality and duration of postoperative analgesia with intraperitoneal instillation of 0.5% bupivacaine after laparoscopic cholecystectomy. Primary outcome measure was Visual Analogue Scale (VAS) score measured at predefined intervals. Secondary outcome measures were the rescue analgesia offered in terms of frequency and total dose.
METHODOLOGY
This prospective, randomized, double blind, placebo controlled study was conducted at Bhopal Memorial Hospital Bhopal, which is a tertiary care centre, between June 2008 – June 2009. After approval by the Hospital Ethics Committee, 60 ASA grade I and II female patients, between 20-60 yrs of age, undergoing elective laparoscopic cholecystectomy under general anesthesia, were enrolled for the study. Written informed consent was obtained after due counseling. The patients who refused to cooperate, were unable to understand or respond to VAS, were allergic to local anesthetics, had chronic pain disease other than gall stone disease, had acute cholecystitis before the operation, choledocholithiasis, acute pancreatitis, or were pregnant, were excluded from the study. Patients were familiarized with visual analogue scale, which consisted of a 10 cm line marked 0-10 at equal intervals, where 0 represented no pain and 10 represented maximum possible pain. Demographic characteristics of patients and American Society of Anesthesiologists (ASA) status were noted. Intensity of pain measured by VAS score was the primary outcome measure.The sample size was based on previous literature.10 Post-hoc power analysis was carried out for level of analgesia (VAS score) at 0, 2 and 4 hours. This study had 81% power to detect effect size of 0.75 between Group S and Group B at the end of 4 hrs; power of 75% to detect effect size of 0.61 between Group S and Group B at the end of 2 hrs and power of 92% to detect effect size of 1.65 between Group S and Group B at 0 hrs; assuming alpha error 0.05.
Randomization was done by assistants by sequentially numbered opaque sealed envelope method and subjects were allocated into two groups of 30 patients each (n=30):
Group S (Placebo group) – patients who were to receive 20 ml of normal saline intraperitoneally after gall bladder extraction
Group B (Study group) – patients who were to receive 20 ml of 0.5% bupivacaine intraperitoneally after gall bladder extraction.
Solutions to be instilled were prepared and coded by an assistant who was unaware of patients grouping during the surgical procedure as well as in the postoperative assessment thus ensuring blinding. Standard fasting and premedication guidelines were followed and a uniform anesthesia and surgical protocol was ensured for all patients. After securing intravenous access, necessary monitors were secured (NIBP, ECG, SpO2, end tidal carbon dioxide (EtCO2) (Philips Medical Systems VM Sure Sign USA). Patients were induced with intravenous midazolam (0.05 mg/kg), fentanyl (1.5-2 microgram/kg) and propofol (1.5-2 mg/kg) and tracheal intubation facilitated with inj. atracurium (0.5 mg/kg). Anesthesia was maintained with isoflurane 1 to 2 % (end tidal concentration) in oxygen and nitrous oxide. Any further analgesia was restricted to intravenous fentanyl 25 µg. Ventilation was adjusted to maintain EtCO2 between 35 to 40 mmHg and the intra-abdominal pressure was maintained between 10 to 12 mmHg. Adequate hydration of patients was ensured during surgery and in the postoperative phase. Inj. ondansetron 4 mg IV was administered after gall bladder extraction. Muscle relaxation was reversed with neostigmine (0.05 mg/kg) and glycopyrrolate (0.01 mg/kg). Any complication arising during or after surgery was managed accordingly. After removal of gall bladder, the surgeon sprayed the given solution through the trocar over the right subdiaphragmatic area, the gall bladder bed and the hepatoduodenal ligament under direct vision and patients were placed in 20º Trendelenburg position for next 5 minutes. CO2 was then evacuated by manual compression of the abdomen through open ports. Time when patient reached recovery room was considered as the 0hour. Total intraoperative dose of intravenous fentanyl (F) for each patient, duration of surgery from surgical incision to skin closure (t1), time interval between administration of last dose of fentanyl and 0 hour (t2), time interval between intraperitoneal drug instillation and 0 hour (t3) were noted. Postoperatively pain parameters were noted at 0, 1, 2, 4, 6, 8, 12 and 24 hours by an observer who was unaware of the solution instilled. Parameters assessed were: intensity of pain at rest (irrespective of location and type of pain) using VAS score, frequency of analgesic administration and total analgesic drug administered. The highest VAS score for each interval between two interviews was noted and analysed at the above mentioned times. Inj diclofenac sodium 75 mg intramuscularly was given as first line rescue analgesic at VAS>4. Inj tramadol 50 mg was administered to any patient who still demanded analgesia or scored >4 on VAS after 20 min of administration of first line analgesia till next 4 hours. Heart rate, mean arterial pressure and respiratory rate were also recorded at corresponding hours. Any other postoperative problem was dealt with accordingly. Data were collected and analyzed using a computer and commercial software (Microsoft Excel). Descriptive statistics was given by mean and standard deviation and proportions wherever appropriate. Two way repeated ANOVA test was used for studying inter group variation in different parameters over time. Pearson chi-square test and unpaired ‘t’ test was used to analyze differences in categorical and numerical variables respectively. A p value <0.05 was considered significant.
Result
60 patients were included in this study with 30 patients in each group. Both groups were comparable as regards demographics and preoperative data (Table 1).
Table 1: Demographics of patients in two groups
|
Characteristics |
Group S |
Group B |
|
Age (years) |
45.4±9.6* |
46.5±10.6* |
|
Weight (kg) |
57.6±10.1* |
58.2±11.0* |
|
ASA grade 1 |
20 |
18 |
|
2 |
10 |
12 |
Group S: 20 ml of normal saline intraperitoneally after gall bladder extraction
Group B: 20 ml of 0.5% bupivacaine intraperitoneally after gall bladder extraction.
* Mean ± SD
There was no statistically significant difference between groups in the total intraoperative dose of fentanyl (F), duration of surgery (t1) in minutes, interval between last dose of intraoperative fentanyl and 0 hour (t2) and interval between intraperitoneal instillation of solutions and 0 hour (t3 ) (Table 2).
Table 2: Intraoperative variables in two groups
|
Parameter |
Group S |
Group B |
p value |
|
F(micrograms) |
127.5 ± 12.0 |
126.7 ± 13.0 |
>0.05 |
|
t1 (min) |
59.8 ± 14.3 |
59.3 ± 14.1 |
>0.05 |
|
t2 (min) |
39.5 ± 7.0 |
41.6 ± 7.0 |
>0.05 |
|
t3 (min) |
22.2 ± 3.3 |
23.6 ± 4.6 |
>0.05 |
F=total intraoperative dose of fentanyl,
t1= duration of surgery (incision to closure)
t2= interval between last dose of fentanyl and 0th hour
t3= interval between intraperitoneal instillation of drug and 0th hour
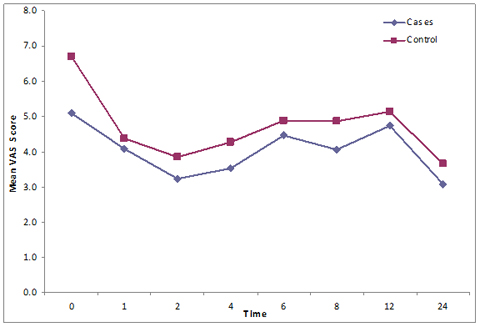
The mean VAS score was less in Group B compared to Group S at each point of analysis. (Figure 1)(Table 3) with a significant decrease (p < 0.05) at 0, 2, 4,8,12 and 24 hours.
Figure I: Comparison of VAS scores between groups
Inter group comparison of VAS scores showed a significantly greater decline in VAS score between 1st and 2nd hour in Group B as compared to Group S (Repeated ANOVA test).The pattern of change in VAS score over time was significantly different (p value <0.05). Frequency of analgesic consumption (3.97 ± 0.85 and 3.93 ± 0.83) during first 24 hours postoperatively did not show any significant difference in the two groups (unpaired ‘t’ test) (Table 3).
Table 3: Comparison of VAS at different time intervals
|
Time intervals (in hours) |
Group S |
Group B |
P value |
|
0 |
6.75 ± 1.66 |
5.10 ± 2.11 |
0.001 |
|
1 |
4.37 ± 0.71 |
4.08 ± 1.18 |
0.264 |
|
2 |
3.83 ± 0.56 |
3.22 ±1.13 |
0.010 |
|
4 |
4.27 ± 0.78 |
3.52 ± 1.22 |
0.006 |
|
6 |
4.88 ± 0.85 |
4.45 ± 0.88 |
0.058 |
|
8 |
4.81 ± 1.08 |
4.05 ± 1.33 |
0.013 |
|
12 |
5.13 ± 0.78 |
4.73 ± 0.63 |
0.032 |
|
24 |
3.65 ± 0.54 |
3.07 ± 0.82 |
0.002 |
|
Frequency of Analgesic requirement |
3.97 ± 0.85 |
3.93 ± 0.83 |
>0.05 |
DISCUSSION
Our study demonstrates that intraperitoneal infiltration of 20 ml of 0.5% bupivacaine produces a significant decline in pain scores in the postoperative phase. Using the same concentration and volume, Bhardwaj et al10 observed significant difference in VAS for 8 hours postoperatively. No significant difference was observed by Jirantarat et al.11 However pain scores in their study could possibly be unreliable because postoperative rescue analgesic was not given based on the VAS score but given “on demand” which includes an element of subjectivity in the results. But they did report that patients in control group requested “rescue” more quickly than study group, suggesting some analgesic action of bupivacaine. Bjorn-Ake-Elfberg et al12 did not observe a statistically significant difference in pain scores in study group, but did observe a better pain relief in heavier patients. However, they have not mentioned the volume and concentration of drug used, or the requirement if any of rescue analgesia, due to which their findings cannot be commented upon. Studies using lower concentrations of study drug14-16 have, however, not reported significant decrease in pain scores after bupivacaine instillation. The beneficial effects demonstrated in our study, may be due to the use of higher concentration of bupivacaine, emphasizing the importance of use of appropriate concentration, volume and total dose of local anesthetic agent for analgesia.
Raetzell et al14 used a lower (0.25%) concentration of bupivacaine, but started postoperative analgesia immediately. No reliance can be placed on pain scores in this study. To refute this fallacy, we recorded pain intensity immediately after shifting the patient to recovery at 0 hour and before administrating post operative rescue analgesia thus eliminating the confounding effect of analgesic action on pain intensity reporting. Many authors who instilled lower concentrations of the drug before dissection of the gall bladder, have observed a significantly better postoperative analgesia comparable with studies using higher concentrations. Szem et al17 suggested that when the drug is instilled after the dissection, incompletely aspirated saline irrigant used during surgery may dilute or disperse the drug from the operative field below an effective concentration, or clotted blood in the field might also interfere with action of the drug. Early instillation also has the advantage of allowing sufficient time for onset of action of drug before emergence from anesthesia. In our study, though the drug was administered after dissection of gall bladder, time between intraperitoneal instillation of drug and first assessment of post operative pain at 0 hour (t3) was adequate for drug action to start. For effective pain reduction at the 0 hour, 0 hour should be considered beyond the time required for onset of the action of bupivacaine. The maximum plasma concentration of bupivacaine after intraperitoneal application of 50 or 100 mg occurs after 5-30 minutes with a mean of 0.48 and 1 mg/L, similar to other techniques of regional anesthesia such as brachial plexus or epidural block.18 In our study also, the mean duration between instillation of drug and 0 hour was 23.6 minutes.
The duration of action of intraperitoneal bupivacaine has been found to vary. Various studies report results ranging from considerable pain reduction 24-48 hours after surgery19,20 to no significant pain reduction.21,22 Other authors detected a modest pain reduction that was detectable only 2 hours after surgery23 or upto 6-8 hours after surgery.24,25 In our study, though a sharper decline in pain scores was seen in bupivacaine group during first 2 hours a significant decrease in pain score compared to saline group lasting for 24 hours after surgery was seen. A local action of local anesthetic along with its absorption from the large surface area, could be an added mechanism of action accounting for this phenomenon.26 Our study does not expound on drug effect in terms of different components of pain viz somatic, visceral and shoulder tip pain. Other studies have commented on the predominance of visceral16,21 and incisional27 pain after laparoscopic cholecystectomy and concluded that intraperitoneal bupivacaine is not effective in blocking these components. However, in these studies Trendelenburg position was not maintained after bupivacaine instillation,21due to which it is possible that the anatomic intraperitoneal flux must have prevented the flow of drug over celiac plexus and phrenic nerve endings thus failing to block visceral pain. In present study, patients were kept in Trendelenberg position after instillation of drug. Shoulder tip pain was the chief complaint on the post operative day1.6 Residual gas can be a prominent cause for this. Due care was taken that carbon dioxide was meticulously evacuated in all our patients to remove this influence. The comparable frequency of analgesic consumption in our study during first 24 hours postoperatively in the two groups (3.97 ± 0.85 in saline group vs. 3.93 ± 0.83 in study group) inspite of a significantly decreased pain intensity in the bupivacaine group is perplexing. These findings are similar to those seen by Chundrigar et al.23 We cannot explain this phenomena apart from the reasoning that an overall decreased frequency of demand for rescue analgesia in both groups may have led to this finding. Additionally, the sometimes-reflexive administration of analgesic drug in the postanesthesia care unit by nursing staff may have masked possible differences in medication use. The total amount of analgesic consumed in the first 24 hours period was also not significantly different between groups. This may be explained by the fact that the local anesthetic had its effects only over the initial few hours, while administration of analgesia was measured over a period far in excess of this timescale, which questions the reliability of analgesic quality of the anesthetic. But inspite of these confounding results, it was evident that the intensity of pain perceived by the patients was less in the bupivacaine group. Any possible bias in recording the pain score was unlikely since we ensured proper concealment of allocation and blinding. The study was carried out in female patients to minimize the biologically based gender differences of pain perception and response to medication.28,29 The visual analogue scale employed in this study has been validated and correlated previously in independent assessments of different types of pain.6 However, pain reporting and scoring is an area of potential discrepancy because of individual differences in the interpretation of an essentially subjective sensation.
It was not possible to measure plasma concentrations of bupivacaine in our cases, though studies have shown that the range of mean plasma concentration (0.99-1.14 µg/ml) after the intraperitoneal administration of plain bupivacaine 100-150 mgis well below the toxic concentrations of 3 µg/ml.14-16 The dose of bupivacaine used in our study was also in this range. Apart from concerns of direct toxicity, Raetzell et al14 have reported postoperative hypoxemic episodes (oxygen saturation <92%) after intraperitoneal bupivacaine. The present study did not evaluate the patient for postoperative pulmonary functions. Use of rescue analgesics in the postoperative phase may also have confounded our results, but the necessity to alleviate patient discomfort took precedence. Lastly, our study was a saline–control study which may lead many to question the results as intraperitoneal saline treatment itself can reduce postoperative pain.30
CONCLUSION
We conclude that intraperitoneal bupivacaine is a simple technique which can be used as a part of multimodal approach to pain control in the postoperative phase after laparoscopic cholecystectomy. Use of the correct dose and concentration of the drug is essential for effective pain control.
REFERENCES
- Schulze S, Thorup J. Cigarini I, et al. Metabolic and respiratory changes after cholecystectomy performed via laparotomy or laparoscopy. Br. J Anaesth. 1992;69:341-5. [PubMed] [Free Full Text]
- Downs SH, Black NA, Devlin HB, et al. Systematic review of the effectiveness and safety of laparoscopic cholecystectomy. Ann R Coll Surg Engl 1996;78:241–323. [PubMed][Free Full Text]
- Bisgaard T, Kehlet H, Rosenberg J. Pain and convalescence after laparoscopic cholecystectomy. Ann R Coll Surg Engl. 2001;167:84–96. [PubMed]
- Bisgaard T, Klarskov B, Rosenberg J, et al. Factors determining convalescence after uncomplicated laparoscopic cholecystectomy. Arch Surg. 2001;136:917–921. [PubMed]
- Wills VL, Hunt DR. Pain after laparoscopic cholecystectomy. Br J Surg. 2000;87:273–284. [PubMed]
- Moiniche S, Jorgensen H, Werrerslev J, Berg J. Local anesthetic infiltration for postoperative pain relief after laparoscopy: a qualitative and quantitative systematic review of intraperitoneal, port-site infiltration and mesosalphinx block. Anest Analg. 2000; 90: 899-91. [PubMed]
- Zmora O, Stolik-Dollberg O, Bar –Zakai B, et al. Intraperitoneal bupivacaine does not attenuate pain following laparoscopic cholecystectomy .JSLS.2000; 4:301-4. [PubMed] [Free Full Text]
- David CW. Analgesic treatment after laparoscopic cholecystectomy. Anesthesiology. 2006;104:835–846. [PubMed][Free Full Text]
- Ahmed B, Ahmed A, Tan D, et al. Post-laparoscopic cholecystectomy pain: effects of intraperitoneal local anesthetics on pain control–a randomized prospective double-blind placebo-controlled trial. Am Surg. 2008;74:201–9.[PubMed]
- Neerja Bhardwaj, Vikas Sharma, Pramila chari. Intraperitoneal bupivacaine instillation for postoperative pain relief after Laparoscopic cholecystectomy. Indian J. Anaesth. 2002;46:49-52.
- Jirantarat V, Rushatakukayanut W, Lert-akyamenee N et al. Analgesic effect of intraperitoneal instillation of bupivacaine for postoperative laparoscopic cholecystectomy. J Med Assoc Thai.Sep 2002;85:S897-903. [PubMed]
- Bjorn-Ake Elfberg and Susanne sjovall-Mjoberg. Intraperitoneal bupicacaine does not effectively reduce pain after Laparoscopic cholecystectomy: A Randomized, Placebo-Controlled and Double blind study. Surg Laparosc Endosc Percutan Tech. 2000;10:357-359. [PubMed]
- Tucker G T, Mather LE. Properties absorption and disposition of loacal anesthetic agents. In:cousins MJ, Bridenbaugh PO, esd. Neural blockade. 2nd ed. Philadelphia: JB Lippincott; 1987.47-110.
- Raetzell M, Maier C, Schröder D, Wulf H. Intraperitoneal application of Bupivacaine during Laparoscopic Cholecystectomy- risk or Benefit. Anesth Analg. 1995;81:967-72. [PubMed]
- Scheinin B, Kellkumpu I, Lindgren L, Haglund C, Rosernberg PH. Effects of intraperitoneal bupivacaine on pain after Laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 1995;39:195-8. [PubMed]
- Joris J, thiry E, Paris P, Lamy M. Pain after Laparoscopic cholecystectomy:
- characteristics and effect of intraperitoneal bupivacaine. Anesth Analg. 1995; 81:379-84. [PubMed]
- Szem J W, Hydo L, Barie PS. A double-blinded evaluation of intraperitoneal bupivacaine v/s saline for the reduction of postoperative pain and nausea after Laparoscopic cholecystectomy. Surg Endosc 1996; 10:44-8. [PubMed]
- Tucker G T, Mather LE. Properties absorption and disposition of loacal anesthetic agents. In:cousins MJ, Bridenbaugh PO, esd. Neural blockade. 2nd ed. Philadelphia: JB Lippincott; 1987.47-110.
- Pasqualucci A, de Angelis V, Contardo R, et al. Preemptive analgesia:intraperitoneal local anaesthetic in Laparoscopic cholecystectomy. A randomized, double blind, placebo controlled study. Anesthesiology. 1996; 85:11-20. [PubMed]
- Weber A, Munoz J, Garteiz D, Cueto J. Use of subdiaphragmatic bupivacaine instillation to control postoperative pain after Laparoscopic surgery. Surg Lparosc Endosc. 1997;7:6-8. [PubMed]
- Rademaker BMP, Kalkman CJ, Odoom JA, de Wit I, Ringers J. intraperitoneal local anaesthetics after Laparoscopic cholecystectomy: effects on postoperative pain, metabolic responses and lung function. Br. J Anaesth. 1994;72:263-6. [PubMed] [Free Full Text]
- Scheinin B, Kellkumpu I, Lindgren L, Haglund C, Rosernberg PH. Effects of intraperitoneal bupivacaine on pain after Laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 1995;39:195-8. [PubMed]
- Chundrigar T, Hedges AR, Morris R, Stamatakis JD. Intraperitoneal bupivacaine for effective pain relief after laparoscopic cholecystectomy. Ann R Coll Engl. 1993; 759:437-9. [PubMed] [Free Full Text]
- Marvoic B, Jurisic T, Kogle-Majeric V, Sustic A. Intraperitoneal bupivacaine for analgesia after Laparoscopic cholecystectomy. Acta Anaesthesiol Scand 1997;41:193-6. [PubMed]
- Tsimoyiannis EC, Glantzounis G, Lekkas ET, et al. Intraperitoneal normal saline and bupivacaine infusion for reduction of postoperative pain after Laparoscopic cholecystectomy. Surg Laparosc Endosc. 1998;8:416-20. [PubMed]
- Ng A, Smith G. Intraperitoneal administration of analgesia: is this practice of any utility? Br J Anaesth .2002;89:4:535-37. [PubMed] [Free Full Text]
- Lee IO, Kim SH, Kong MH, et al. Pain after laparoscopic cholecystectomy: The effect and timing of intraperitoneal bupivacaine. Can J Anesthesia 2001;48:545-50. [PubMed]
- Buckelew S P, Shutty MS Jr, Hewett J, et al. Health locus of control, gender differences and adjustment to persistent pain. Pain 1990;42 :287-294. [PubMed]
- Gear RW, Miaskowski C,Gordon N C, et al .Kappa opioids produce significanty greater analgesia in women than men. Nature Med 1996;2:1248-50. [PubMed]
- Wills VL, Hunt DR. Pain after laparoscopic cholecystectomy. Br J Surg 2000;87:273-84. [PubMed]




 Facebook
Facebook Twitter
Twitter GooglePlus
GooglePlus Youtube
Youtube