Mohammed Hakim, MBBS1, Ralph J. Beltran, MD1,2, Asad A. Khawaja, BA3, Ahsan Syed, MD1,2, Joseph D. Tobias, MD1,2
1Department of Anesthesiology & Pain Medicine, Nationwide Children’s Hospital, Columbus, Ohio, USA
2Department of Anesthesiology & Pain Medicine, The Ohio State University, Columbus, Ohio, USA
3Marshall University, Joan C. Edwards School of Medicine, Huntington, West Virginia, USA
Correspondence: Mohammed Hakim, MBBS, Department of Anesthesiology & Pain Medicine, Nationwide Children's Hospital, 700 Children's Drive, Columbus, Ohio 43205; Phone: (614) 722-3846; FAX: (614) 722-4203; E-mail: Mohammed.Hakim@nationwidechildrens.org
ABSTRACT
Interscalene blockade (ISB) is frequently used for intraoperative anesthesia and postoperative analgesia for surgical procedures involving the shoulder and upper extremity. While generally devoid of adverse effects, inadvertent damage to or spread of the local anesthetic agent to surrounding neurovascular structures in the neck may result in unintended consequences. We present the report of a 21-year-old male, who underwent diagnostic shoulder arthroscopy and labral repair. To supplement general anesthesia and provide postoperative pain, an ISB was placed. The immediate postoperative course was uneventful and the patient was discharged home. A few hours later, the patient complained of numbness over his face that progressed to Horner’s syndrome with prolonged ptosis lasting for 6 months. The potential etiologies of Horner’s syndrome following ISB are presented, previous reports of this complication reviewed, and treatment strategies discussed.
Key words: Interscalene block; Horner’s syndrome; Ptosis
Citation: Hakim M, Beltran RJ, Khawaja AA, Syed A, Tobias JD. Prolonged Horner’s syndrome after intraoperative interscalene block. Anaesth Pain & Intensive Care 2017;21(4):468-471
Received: 27 Oct 2017; Reviewed: 4 Nov 2017; Corrected & Accepted: 14 Dec 2017.
INTRODUCTION
The interscalene approach to the brachial plexus remains a safe and effective technique for providing anesthesia of the upper extremity in both adults and children.1,2 The brachial plexus is derived from the ventral (anterior) branches of spinal roots C5-8 and T1. These spinal nerves pass through the intervertebral foramina, travelling between the anterior and middle scalene muscles. The fascia from the scalene muscles forms a sheath which surrounds the brachial plexus. Also encased in the fascial sheath is the subclavian artery, which continues distally as the axillary artery.3 As the spinal roots pass between the anterior and middle scalene muscles, they unite to form three trunks (superior, middle, and inferior). As the trunks exit the inter-scalene groove, they lie in a cephaloposterior position to the subclavian artery as it courses along the upper surface of the first rib. The trunks split into anterior and posterior divisions which then unite to form cords (lateral, posterior and medial) which are named because of their relationship to the axillary artery. The cords further divide into the musculocutaneous, ulnar, median, and radial nerves. During the interscalene approach to the brachial plexus, inadvertent damage to or spread of the local anesthetic agent to surrounding neurovascular structures in the neck may result in phrenic nerve blockade, recurrent laryngeal nerve blockade or Horner’s syndrome.3-6 Transient Horner’s syndrome from the effect of the local anesthetic agent on the sympathetic chain occurs with a frequency of up to 60-75% with brachial plexus blockade.3,5 This effect resolves as the brachial plexus motor and sensory blockade resolve with dissipation of the effects of the local anesthetic agent. To date, there have been a limited number of reports of prolonged unilateral ptosis following placement of an interscalene block (ISB). We present here a report of a 21-year-old male, who underwent diagnostic shoulder arthroscopy and labral repair with a technique that included general anesthesia supplemented by brachial plexus blockade using an interscalene approach. The potential etiologies of Horner’s syndrome following ISB are presented, previous reports of this complication reviewed, and treatment strategies discussed.
CASE REPORT
Institutional Review Board approval is not required at Nationwide Children’s Hospital (Columbus, Ohio) for the presentation of single case reports. The patient was a 21-year-old, active male weighing 103.5 kg. He presented initially with right shoulder pain while training as a firefighter candidate, and later on presented for a diagnostic arthroscopy and labral repair of his shoulder. The patient’s past anesthesia history included general anesthesia and an ISB for a left shoulder arthroscopic, superior labrum anterior and posterior repair, and rotator cuff debridement two years earlier. He had no known environmental or drug allergies. The remainder of his medical history, preoperative examination and preoperative lab work, were all normal. After the induction of general anesthesia, the airway was secured with a laryngeal mask airway (LMA) and anesthesia maintained with isoflurane in air and oxygen with fentanyl (2 µg/kg). After the right side of the neck and supraclavicular area were prepared with chlorhexidine, a 21 gauge, 4 cm Stimuplex® (B. Braun, Medical, Inc, Bethlehem, PA) needle was introduced under ultrasound guidance. The brachial plexus trunks were identified between the anterior and middle scalene muscles at the level of the cricoid cartilage. Twenty ml of 0.5% ropivacaine with epinephrine 1:200,000 and preservative free dexamethasone (4 mg) was injected. The patient’s postoperative course was uneventful and he was discharged home, having adequate analgesia without any need of additional opioids in the post-anesthesia care unit (PACU). Subsequently, the night after surgery, the regional anesthesia team was contacted as the patient had noted the onset of numbness localized to the right side of his face involving the eye. Although these symptoms resolved overnight, the next morning, the patient noted a “droopy eyelid” which however, did not interfere in his routine daily activities (Figure 1).
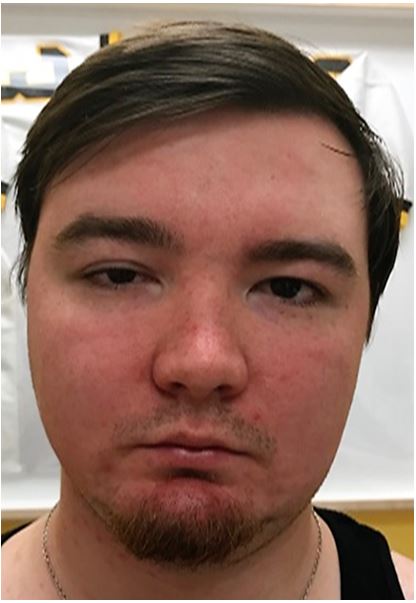
Figure 1: Showing Horner’s syndrome on the right side
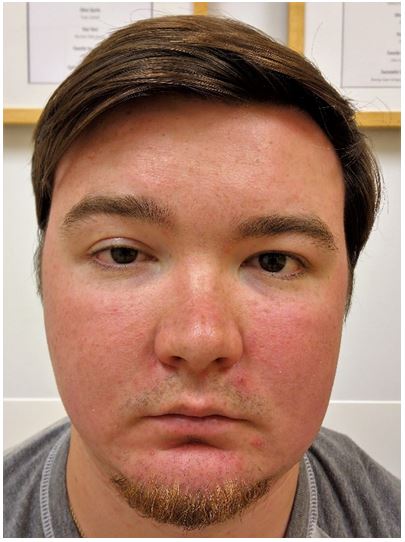
Figure 2: Showing drooping of the right upper eyelid after three months
He denied having neck swelling, neck pain, or swallowing or breathing problems at any time. As the ptosis persisted, the regional anesthesia team was contacted for advice. Magnetic resonance imaging (MRI) of the neck and ultrasonography of the cervical vessels were advised but were found normal. Therefore, it was concluded, that this was a rare and unexpected complication. Recovery times in such cases was estimated to be between 6 months to a year. On the patient’s most recent follow-up approximately 3 months after the surgical procedure, the Horner’s syndrome has resolved, but there was persistent ptosis (Figure 2).
DISCUSSION
The clinical presentation of Horner’s syndrome includes ptosis, miosis (due to interruption of the dilator pupillae muscle), anhydrosis, conjunctival injection, and relative enophthalmos. These clinical signs and symptoms result from the interruption of the normal sympathetic pathways. Ptosis results from the interruption of the sympathetic innervation to Muller’s muscle of the upper eyelid and not from involvement of cranial nerve III (oculomotor nerve).5 As noted in our patient, the symptoms may vary including one or all of the classical components.
Unlike the parasympathetic innervation of the head and neck, the sympathetic nerves exit the central nervous system from the thoracic spinal cord. They must therefore travel cephalad back into the head and neck region to exert their influence. The axons of the first-order neurons of the sympathetic fibers, the cell bodies of which are located in the midbrain (hypothalamus), travel caudal through the brain stem and into the spinal cord to the thoracic level. These fibers do not cross the midline, synapsing in the intermediolateral gray column of the thoracic spinal cord at the level of C7-T3 with the cell body of the second-order neuron. The second-order neuron exits the thoracic spinal cord travelling in white rami communicantes, passing over the apex of the lung within the thoracic cavity, travelling cephalad into the neck through the thoracic sympathetic ganglia (stellate ganglion), synapsing with the cell body of the third-order neuron in the superior cervical ganglion. From there, the third-order neuron (post-ganglionic fibers) travels alongside the great vessels in the neck, enters the cranium, and then the orbit to innervate the eye.
Horner’s syndrome is the result of interruption of or damage to the normal sympathetic pathway, occurring at the level of first-, second- or third-order neuron.7 Damage to the first-order neurons in the midbrain or hypothalamus causes central Horner’s syndrome. Central Horner’s syndrome was unlikely in our patient due to the isolated nature of the symptoms, its temporal association with the ISB, normal MRI, and the lack of other clinical signs of CNS involvement. Apical lung tumors can compress the second-order or pre-ganglionic neurons, while involvement of the third-order neuron occurs in the area near the stellate ganglion and around the great vessels of the neck. The latter is the most likely area involved in our patient, given its proximity to the area of needle insertion and deposition of the local anesthetic agent. History, clinical examination, and pharmacologic testing can help direct appropriate imaging or even render imaging studies unnecessary.1,4 However, in most situations, as with our patient, imaging of the entire three-neuron sympathetic pathway is warranted when the etiology is in question.1
Horner’s syndrome may accompany regional anesthetic techniques including stellate ganglion block and various supraclavicular approaches to the brachial plexus.2,3 The clinical signs and symptoms of Horner’s syndrome are transient, generally paralleling the duration of the local anesthetic effect on sensory and motor function. Prolonged effects as noted in our patient are rare and cannot be attributed to the residual effects of the local anesthetic agent. Other possible etiologies related to therapeutic procedures or co-morbid disease processes include inadvertent trauma to the stellate ganglion and the sympathetic chain during attempts at cannulation of the internal jugular vein, anterior thoracic surgical procedures involving the great vessels, epidural analgesia, and dissection of the internal carotid and vertebral arteries have shown to present with Horner’s syndrome.8-11 Some rare possible causes of Horner’s syndrome include infarcts (such as Wallenberg syndrome), demyelinating diseases, cavernous sinus pathology, zoster, and cluster headaches etc. As other potential etiologies were ruled out and given the close temporal proximity of the ISB to the development of Horner’s syndrome, we postulate that the ISB was the most likely etiology.
Two previous reports outline prolonged Horner’s syndrome after ISB.12,13 As with our patient, the effects were initially attributed to the transient effects of the local anesthetic agent, but then persisted beyond the duration of the motor and sensory blockade. Sukhani et al. reported prolonged Horner’s syndrome following an ISB using a nerve stimulator.12 The clinical symptoms persisted for more than one month, most notably the ptosis. The authors attributed the problem to traumatic injury to the sympathetic chain during placement of the ISB, although they also postulated that chemical injury from the local anesthetic agent was an alternative etiology. However, they felt that the latter was not likely as they had used recommended concentrations and volumes of mepivacaine and tetracaine.
Given the duration of the signs and symptoms in our patient, we would postulate that either traumatic injury to the sympathetic chain occurred during needle insertion for ISB or that a hematoma developed following the procedure. Ultrasound guidance was used in our patient and only a single pass of the needle was required, thereby limiting the potential for inadvertent trauma to surrounding structures. Hematoma formation is an alternative etiology, which has been previously reported following ISB13; however, no findings were found in the imaging studies in our patient.
While Horner’s syndrome poses no severe clinical consequences, it can lead to significant anxiety and psychological impact, given the altered body image related to ptosis. Therefore, it is important to provide education and reassurance while monitoring for resolution of the symptoms. Albeit rare, inadvertent trauma to surrounding structures, such as the sympathetic pathways, may occur with needle insertion and consideration should be given to including such complications in the informed consent process. We continue to recommend the use of ultrasound during regional anesthetic techniques to enhance safety.
In summary, Horner’s syndrome with right sided ptosis following ISB is usually transient and signs and symptoms may occur for a duration related to the effect of the long anesthetic agents. Unexpectedly prolonged duration should prompt us to search for other etiologies. Imaging studies are indicated and are helpful to look for any possible alternative pathology.
Conflict of interest: None declared by the authors
Authors’ contribution:
MH: Wrote the manuscript and submitted
RB: Reviewed and editing the manuscript
AK: Designed the initial manuscript
AS: Performed the procedure and reviewed
JT: Reviewed and approved the final manuscript as submitted
REFERENCES
1Department of Anesthesiology & Pain Medicine, Nationwide Children’s Hospital, Columbus, Ohio, USA
2Department of Anesthesiology & Pain Medicine, The Ohio State University, Columbus, Ohio, USA
3Marshall University, Joan C. Edwards School of Medicine, Huntington, West Virginia, USA
Correspondence: Mohammed Hakim, MBBS, Department of Anesthesiology & Pain Medicine, Nationwide Children's Hospital, 700 Children's Drive, Columbus, Ohio 43205; Phone: (614) 722-3846; FAX: (614) 722-4203; E-mail: Mohammed.Hakim@nationwidechildrens.org
ABSTRACT
Interscalene blockade (ISB) is frequently used for intraoperative anesthesia and postoperative analgesia for surgical procedures involving the shoulder and upper extremity. While generally devoid of adverse effects, inadvertent damage to or spread of the local anesthetic agent to surrounding neurovascular structures in the neck may result in unintended consequences. We present the report of a 21-year-old male, who underwent diagnostic shoulder arthroscopy and labral repair. To supplement general anesthesia and provide postoperative pain, an ISB was placed. The immediate postoperative course was uneventful and the patient was discharged home. A few hours later, the patient complained of numbness over his face that progressed to Horner’s syndrome with prolonged ptosis lasting for 6 months. The potential etiologies of Horner’s syndrome following ISB are presented, previous reports of this complication reviewed, and treatment strategies discussed.
Key words: Interscalene block; Horner’s syndrome; Ptosis
Citation: Hakim M, Beltran RJ, Khawaja AA, Syed A, Tobias JD. Prolonged Horner’s syndrome after intraoperative interscalene block. Anaesth Pain & Intensive Care 2017;21(4):468-471
Received: 27 Oct 2017; Reviewed: 4 Nov 2017; Corrected & Accepted: 14 Dec 2017.
INTRODUCTION
The interscalene approach to the brachial plexus remains a safe and effective technique for providing anesthesia of the upper extremity in both adults and children.1,2 The brachial plexus is derived from the ventral (anterior) branches of spinal roots C5-8 and T1. These spinal nerves pass through the intervertebral foramina, travelling between the anterior and middle scalene muscles. The fascia from the scalene muscles forms a sheath which surrounds the brachial plexus. Also encased in the fascial sheath is the subclavian artery, which continues distally as the axillary artery.3 As the spinal roots pass between the anterior and middle scalene muscles, they unite to form three trunks (superior, middle, and inferior). As the trunks exit the inter-scalene groove, they lie in a cephaloposterior position to the subclavian artery as it courses along the upper surface of the first rib. The trunks split into anterior and posterior divisions which then unite to form cords (lateral, posterior and medial) which are named because of their relationship to the axillary artery. The cords further divide into the musculocutaneous, ulnar, median, and radial nerves. During the interscalene approach to the brachial plexus, inadvertent damage to or spread of the local anesthetic agent to surrounding neurovascular structures in the neck may result in phrenic nerve blockade, recurrent laryngeal nerve blockade or Horner’s syndrome.3-6 Transient Horner’s syndrome from the effect of the local anesthetic agent on the sympathetic chain occurs with a frequency of up to 60-75% with brachial plexus blockade.3,5 This effect resolves as the brachial plexus motor and sensory blockade resolve with dissipation of the effects of the local anesthetic agent. To date, there have been a limited number of reports of prolonged unilateral ptosis following placement of an interscalene block (ISB). We present here a report of a 21-year-old male, who underwent diagnostic shoulder arthroscopy and labral repair with a technique that included general anesthesia supplemented by brachial plexus blockade using an interscalene approach. The potential etiologies of Horner’s syndrome following ISB are presented, previous reports of this complication reviewed, and treatment strategies discussed.
CASE REPORT
Institutional Review Board approval is not required at Nationwide Children’s Hospital (Columbus, Ohio) for the presentation of single case reports. The patient was a 21-year-old, active male weighing 103.5 kg. He presented initially with right shoulder pain while training as a firefighter candidate, and later on presented for a diagnostic arthroscopy and labral repair of his shoulder. The patient’s past anesthesia history included general anesthesia and an ISB for a left shoulder arthroscopic, superior labrum anterior and posterior repair, and rotator cuff debridement two years earlier. He had no known environmental or drug allergies. The remainder of his medical history, preoperative examination and preoperative lab work, were all normal. After the induction of general anesthesia, the airway was secured with a laryngeal mask airway (LMA) and anesthesia maintained with isoflurane in air and oxygen with fentanyl (2 µg/kg). After the right side of the neck and supraclavicular area were prepared with chlorhexidine, a 21 gauge, 4 cm Stimuplex® (B. Braun, Medical, Inc, Bethlehem, PA) needle was introduced under ultrasound guidance. The brachial plexus trunks were identified between the anterior and middle scalene muscles at the level of the cricoid cartilage. Twenty ml of 0.5% ropivacaine with epinephrine 1:200,000 and preservative free dexamethasone (4 mg) was injected. The patient’s postoperative course was uneventful and he was discharged home, having adequate analgesia without any need of additional opioids in the post-anesthesia care unit (PACU). Subsequently, the night after surgery, the regional anesthesia team was contacted as the patient had noted the onset of numbness localized to the right side of his face involving the eye. Although these symptoms resolved overnight, the next morning, the patient noted a “droopy eyelid” which however, did not interfere in his routine daily activities (Figure 1).

Figure 1: Showing Horner’s syndrome on the right side

Figure 2: Showing drooping of the right upper eyelid after three months
He denied having neck swelling, neck pain, or swallowing or breathing problems at any time. As the ptosis persisted, the regional anesthesia team was contacted for advice. Magnetic resonance imaging (MRI) of the neck and ultrasonography of the cervical vessels were advised but were found normal. Therefore, it was concluded, that this was a rare and unexpected complication. Recovery times in such cases was estimated to be between 6 months to a year. On the patient’s most recent follow-up approximately 3 months after the surgical procedure, the Horner’s syndrome has resolved, but there was persistent ptosis (Figure 2).
DISCUSSION
The clinical presentation of Horner’s syndrome includes ptosis, miosis (due to interruption of the dilator pupillae muscle), anhydrosis, conjunctival injection, and relative enophthalmos. These clinical signs and symptoms result from the interruption of the normal sympathetic pathways. Ptosis results from the interruption of the sympathetic innervation to Muller’s muscle of the upper eyelid and not from involvement of cranial nerve III (oculomotor nerve).5 As noted in our patient, the symptoms may vary including one or all of the classical components.
Unlike the parasympathetic innervation of the head and neck, the sympathetic nerves exit the central nervous system from the thoracic spinal cord. They must therefore travel cephalad back into the head and neck region to exert their influence. The axons of the first-order neurons of the sympathetic fibers, the cell bodies of which are located in the midbrain (hypothalamus), travel caudal through the brain stem and into the spinal cord to the thoracic level. These fibers do not cross the midline, synapsing in the intermediolateral gray column of the thoracic spinal cord at the level of C7-T3 with the cell body of the second-order neuron. The second-order neuron exits the thoracic spinal cord travelling in white rami communicantes, passing over the apex of the lung within the thoracic cavity, travelling cephalad into the neck through the thoracic sympathetic ganglia (stellate ganglion), synapsing with the cell body of the third-order neuron in the superior cervical ganglion. From there, the third-order neuron (post-ganglionic fibers) travels alongside the great vessels in the neck, enters the cranium, and then the orbit to innervate the eye.
Horner’s syndrome is the result of interruption of or damage to the normal sympathetic pathway, occurring at the level of first-, second- or third-order neuron.7 Damage to the first-order neurons in the midbrain or hypothalamus causes central Horner’s syndrome. Central Horner’s syndrome was unlikely in our patient due to the isolated nature of the symptoms, its temporal association with the ISB, normal MRI, and the lack of other clinical signs of CNS involvement. Apical lung tumors can compress the second-order or pre-ganglionic neurons, while involvement of the third-order neuron occurs in the area near the stellate ganglion and around the great vessels of the neck. The latter is the most likely area involved in our patient, given its proximity to the area of needle insertion and deposition of the local anesthetic agent. History, clinical examination, and pharmacologic testing can help direct appropriate imaging or even render imaging studies unnecessary.1,4 However, in most situations, as with our patient, imaging of the entire three-neuron sympathetic pathway is warranted when the etiology is in question.1
Horner’s syndrome may accompany regional anesthetic techniques including stellate ganglion block and various supraclavicular approaches to the brachial plexus.2,3 The clinical signs and symptoms of Horner’s syndrome are transient, generally paralleling the duration of the local anesthetic effect on sensory and motor function. Prolonged effects as noted in our patient are rare and cannot be attributed to the residual effects of the local anesthetic agent. Other possible etiologies related to therapeutic procedures or co-morbid disease processes include inadvertent trauma to the stellate ganglion and the sympathetic chain during attempts at cannulation of the internal jugular vein, anterior thoracic surgical procedures involving the great vessels, epidural analgesia, and dissection of the internal carotid and vertebral arteries have shown to present with Horner’s syndrome.8-11 Some rare possible causes of Horner’s syndrome include infarcts (such as Wallenberg syndrome), demyelinating diseases, cavernous sinus pathology, zoster, and cluster headaches etc. As other potential etiologies were ruled out and given the close temporal proximity of the ISB to the development of Horner’s syndrome, we postulate that the ISB was the most likely etiology.
Two previous reports outline prolonged Horner’s syndrome after ISB.12,13 As with our patient, the effects were initially attributed to the transient effects of the local anesthetic agent, but then persisted beyond the duration of the motor and sensory blockade. Sukhani et al. reported prolonged Horner’s syndrome following an ISB using a nerve stimulator.12 The clinical symptoms persisted for more than one month, most notably the ptosis. The authors attributed the problem to traumatic injury to the sympathetic chain during placement of the ISB, although they also postulated that chemical injury from the local anesthetic agent was an alternative etiology. However, they felt that the latter was not likely as they had used recommended concentrations and volumes of mepivacaine and tetracaine.
Given the duration of the signs and symptoms in our patient, we would postulate that either traumatic injury to the sympathetic chain occurred during needle insertion for ISB or that a hematoma developed following the procedure. Ultrasound guidance was used in our patient and only a single pass of the needle was required, thereby limiting the potential for inadvertent trauma to surrounding structures. Hematoma formation is an alternative etiology, which has been previously reported following ISB13; however, no findings were found in the imaging studies in our patient.
While Horner’s syndrome poses no severe clinical consequences, it can lead to significant anxiety and psychological impact, given the altered body image related to ptosis. Therefore, it is important to provide education and reassurance while monitoring for resolution of the symptoms. Albeit rare, inadvertent trauma to surrounding structures, such as the sympathetic pathways, may occur with needle insertion and consideration should be given to including such complications in the informed consent process. We continue to recommend the use of ultrasound during regional anesthetic techniques to enhance safety.
In summary, Horner’s syndrome with right sided ptosis following ISB is usually transient and signs and symptoms may occur for a duration related to the effect of the long anesthetic agents. Unexpectedly prolonged duration should prompt us to search for other etiologies. Imaging studies are indicated and are helpful to look for any possible alternative pathology.
Conflict of interest: None declared by the authors
Authors’ contribution:
MH: Wrote the manuscript and submitted
RB: Reviewed and editing the manuscript
AK: Designed the initial manuscript
AS: Performed the procedure and reviewed
JT: Reviewed and approved the final manuscript as submitted
REFERENCES
- Winnie AP. InterscaIene brachial plexus block. Anesth Analg 1970;49:455-66. [PubMed]
- Tobias JD. Brachial plexus anesthesia in children. Paediatr Anaesth 2001;11:265-75. [PubMed] [Free full text]
- Hickey R, Garland TA, Ramaurthy S. Subclavian perivascular block influence of location of paresthesia. Anesth Analg 1989;68:767-71. [PubMed]
- Urmey WF, Talts KH, Sharrock NE. One hundred percent incidence of hemidiaphragmatic paresis associated with interscalene brachial plexus anesthesia as diagnosed by ultrasonography. Anesth Analg 1991;72:498-503. [PubMed]
- Lenters TR, Davies J, Matsen FA 3rd. The types and severity of complications associated with interscalene brachial plexus block anesthesia: Local and national evidence. J Shoulder Elbow Surg 2007;16:379-87. [PubMed]
- Vester-Andersen T, Christiansen C, Hansen A, Sørensen M, Meisler C. Interscalene brachial plexus block: Area of analgesia, complications and blood concentrations of local anesthetics. Acta Anaesthesiol Scand 1981;25:81-4. [PubMed]
- Lee JH, Lee HK, Lee DH, Choi CG, Kim SJ, Suh DC. Neuroimaging strategies for three types of Horner syndrome with emphasis on anatomic location. Am J Roentgenol 2007;188:W74-81. [PubMed]
- Tobias JD, Joy BF. Horner syndrome following attempted internal jugular catheter placement in a toddler with congenital heart disease. J Med Cases 2014;5:369-72.
- Kaya SO, Liman ST, Bir LS, et al. Horner’s syndrome as a complication in thoracic surgical practice. Eur J Cardiothorac Surg 2003;24:1025-8.
- Clayton KC. The incidence of Horner’s syndrome during lumbar extradural for elective Caesarean section and provision of analgesia during labour. Anaesthesia 1983;38:583-5. [PubMed] [Free full text]
- Lyrer PA, Brandt T, Metso TM, et al. Clinical import of Horner syndrome in internal carotid and vertebral artery dissection. Neurology2014;82:1653-9. doi: 10.1212/WNL.0000000000000381[PubMed] [Free full text]
- Sukhani R, Barclay J, Aasen M. Prolonged Horner’s syndrome after interscalene block: A management dilemma. Anesth Analg 1994;79;601-3. [PubMed]
- Ekatodramis G, Macaire P, Borgeat A. Prolonged Horner syndrome due to neck hematoma after continuous interscalene block. Anesthesiology 2001;95;801-3. [PubMed] [Free full text]