*Assistant Professor; **Professor & Head; ***Resident
Department of Anesthesiology, Pad. Dr. D Y Patil Medical College, Pimpri, Pune, Maharashtra (India)
Correspondence: Dr. Aparna Girwalkar-Bagle, Assistant Professor, Department of Anesthesiology, Pad. Dr. D Y Patil Medical College, Pimpri, Pune, Maharashtra (India)
ABSTRACT
Sacrococcygeal teratoma is a tumour located at the base of coccyx (tail bone) and thought to be derived from embryonic germ cell layers.¹̛ ² The tumors present mostly in infancy and are extremely rare in adults. Modern imaging techniques may be helpful to detect the extent of mass, but surgical excision is generally indicated at the time of detection. Most sacrococcygeal teratomas are not likely to be malignant and prognosis tends to be good after resection. Here we report a case and anesthetic / surgical management of a huge sacrococcygeal teratoma, which was more than half of the size and weight of the baby.
Key words: Sacrococcygeal teratoma; AnesthesiaCitation: Girwalkar-Bagle A, Thatte WS, Gulia P. Sacrococcygeal teratoma: A case report and review of literature. Anaesth Pain & Intensive Care 2014;18(4):449-51
INTRODUCTIONSacrococcygeal teratoma (SCT) is rare and happens in 1:35,000 to 40,000 live births. It is more common in girls than boys. Girls to boys ratio of 3:1 to 4:1 have been reported.3,4 The etiology is unknown. The tumors are composed of two or three germ cell layers, so usually have multiple tissue types. Most SCT are found in neonates, infants and children below 4 yrs but have been reported in adult also. The tumor usually has both solid and cystic (fluid filled) parts. Some of the solid tumors have very large blood supply. Due to high vascularity can cause problem during pregnancy and the postoperative period. Like other teratomas SCT can grow very large even larger than the size of the baby. The tumors that are found in new born period are usually benign but few can be malignant.⁵̛ ⁶
A fetus with SCT remains at high risk of perinatal complications and have higher mortality rates due to maternal obstetric complications such as tumour rupture⁷, preterm labour or dystocia.The fetus is also at risk of high output cardiac failure⁸, placentomegally and hydrops with subsequent fetal demise secondary to the increased metabolic demands and vascular steal of a rapidly growing solid tumour⁸̛ ⁹. Modern technology using three dimensional (3D) sonography now allows the prenatal diagnosis of SCT even in the first trimester¹°̛ ¹¹. Surgical resection remains the mainstay of management¹² and after complete surgical excision recurrence is rare. This case is reported to highlight the clinical presentation,anesthetic and surgical management of SCT in premature neonate.
CASE REPORTA 23 years old primigravida with 33 weeks gestation referred to our institution after sonographic finding of sacrococcygeal mass 9.5 cm X 10 cm for further management. She had adequate amniotic fluid and other parameters (head circumference, femur length, cardiac activity) corresponding to the gestational age was within normal limits. It was her first antenatal visit. She was hemodynamically stable and all biochemical investigations (hemogram, complete blood count, renal function tests, kidney function tests, blood sugar, urine examination,PT/INR and ECG) were normal. After admission in obstetric ward on second day she went into labor. In view of large sacrococcygeal teratoma she underwent emergency cesarean section under spinal anesthesia. A baby girl was delivered with a good cry and with a large SCT 10 X 10 cms arising from sacral region (Fig 1). Dilated veins on the swelling could be seen. (Fig 2). Baby weighed 2.5 kg and was stable with good power in all four limbs. She was shifted to NICU for monitoring and in view of prematurity. X-ray and MRI spine was done (Fig 3) showing clear demarcation between tumour and spine.
After 1 week baby posted for SCT excision. All investigations were within normal limits (Hb 13 gm%, serum creatinine 1.1 mg/dl, BUN 29 mg/dl, blood sugar 100 mg/dl, bilirubin 1.2 mg/dl, SGOT 22 IU/dl, SGPT 23 IU/dl, serum alkaline phosphatase 112 IU/dl, PT 14(15), INR 1, serum electrolytes and urine examination normal. Whole body x-ray was ordered to rule out any other congenital anomaly and bone involvement of SCT, and was found to be within normal limit. 2D Echo showed tiny patent ductus arteriosus and mild LV dysfunction. USG abdomen was within normal limits and no bowel or bladder involvement. From radiological investigations it was seen to be arising from coccyx and was labelled as Type 1 SCT with mass effect on rectum.
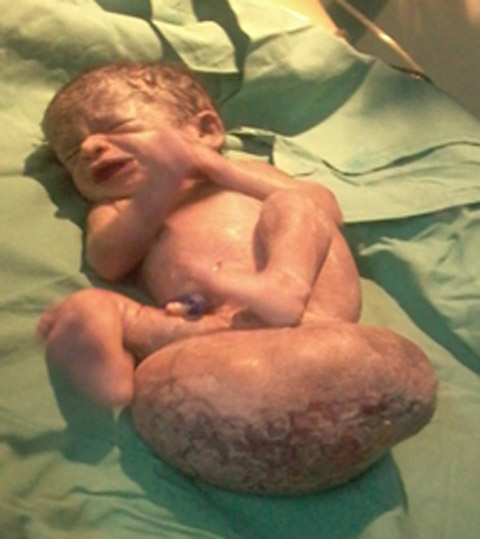
Fig 1: Showing the large sacrcoccygeal teratoma
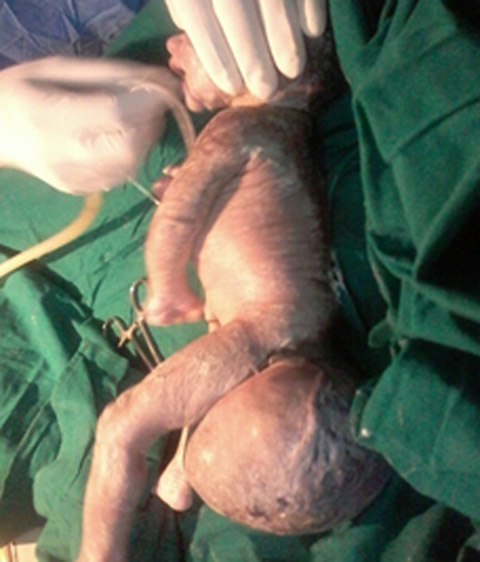
Fig 2: showing lateral aspect of baby and SCT with dilated veins
Baby was kept nil by mouth for four hours prior to operation, and she received maintenance fluid 10 ml/hr. She was premedicated with 0.01 mg glycopyrrolate and fentanyl 5 microgram IV. Preoxygenation was done for 3 min. Before induction baby was placed on soft pillow with tumour supported with soft cotton rolls beneath and around. Induction was done with sevoflurane and baby was intubated with endotracheal tube size 2.5 mm. Anesthesia was maintained with sevoflurane 1-2% in oxygen and nitrous oxide. Muscle relaxation was achieved with atracurium 1.25 mg IV given as loading dose followed by top ups of 0.25 mg. Baby was kept warm by wrapping limbs and head in cotton rolls. ECG, HR and SpO2 was monitored. Surgical resection of tumor including coccygectomy was done. At the end of surgery her spontaneous respiratory attempts were good, so reversed with neostigmine 0.125 mg and glycopyrrolate 0.02 mg. After regaining adequate muscle tone, cough and gag reflex, she was extubated. Intraoperative blood loss was replaced with 70 ml of packed cell volume. Postoperatively baby shifted to NICU for further monitoring and management. In NICU baby received oxygen with hood and dobutamine infusion 2.5 microgram/kg/min (in view of low volume pulse and mild LV dysfunction on Echo) for 24 hours. The baby had an uneventful postoperative recovery and was discharged home after 15 days.
DISCUSSIONThe newborn with SCT has an excellent prognosis depending on the time of diagnosis, malignant potential of the tumour and the ease of surgical excision.¹² Early prenatal diagnosis is possible. SCT could be diagnosed from second trimester of pregnancy when there is polyhydraminos and/or uterus larger than the gestational age. Prenatal diagnosis is of significance, since early prenatal presentation is associated with high fetal morbidity and mortality while presentation after 30 weeks is a relatively good prognostic indicator for fetal survival.¹⁴
Monitoring for fetal distress during pregnancy is very important. Some large tumors have a very high blood flow that causes a shift in blood flow away from the baby towards the tumour. As it grows it can cause the baby to become sick and hydropic. This means the heart begins to fail and the baby becomes swollen. Other possible complications include bleeding inside the tumour, development of excess amniotic fluid and preterm labour. Progressive hydrops can be associated with a swollen and sick placenta. There is a rare condition called ‘Mirror syndrome’, where the mother mirrors the baby’s sickness.13 This is due to fluid retention in fetal compartments, water retention in mother also occurs and she suffers the same symptoms as the sick fetus. Mother will become ill and have signs of preeclampsia, water retention, high blood pressure, protein in the urine, placetomegally and failing heart. If this occurs baby should be delivered immediately.
Early diagnosis may necessitate delivery by cesarean section in centers with good neonatal facilities where early surgical treatment can be offered to the baby.14 That’s what happened with our case due to prenatal diagnosis baby delivered safely with cesarean section and also surgical excision was done at earliest. Similarly fetal surgical procedures15 could be undertaken when the diagnosis is made early in the pregnancy. Well planned surgical excision including coccyx excision was done in order to forestall possible recurrence. Anesthetic challenges were mainly prematurity and blood loss. Atracurium was used for muscle relaxation considering prematurity of organs. Blood loss was replaced with blood (packed cell volume) immediately. Adequate pain relief was provided.
Complete excision including coccygectomy is the primary therapy for all SCT and it is adequate⁴̛ ⁵̛ ¹⁶ if the tumor is benign. Altman type 1 SCT, meticulous anesthetic and surgical management, good postoperative monitoring and care in neonatal intensive care unit and benign nature of the tumor all these things contributed to the miraculous outcome of the baby.
Extensive surgery in the pelvis and perineal region may involve disruption of nerves and muscles which supply urinary/anorectal sphincters and provide maximum support in normal working respectively. Alpha fetoprotein well known marker of teratomas, is valuable in differentiating between mature and malignant teratomas at presentation and during follow up of patients.16,17 It may be utilized to detect early occurrence of malignancy. This is not only appropriate during first three postoperative years when recurrence is likely, but on long term basis as significant number of them suffer from deficient anorectal function and diminished quality of life.¹⁶
REFERENCES- Isaacs H, Jr. Perinatal (fetal and neonatal) germ cell tumours. J of Pediatric Surgery 2004;39:1003-13. [PubMed]
- Ashley DJ. Origin of teratomas. Cancer 1973;32:390-4. [PubMed]
- Shonubi AMO, Musa AA, Akiode O, Salami. Mature Sacrococcygeal Teratomas: A Case Report and literature review. West Afri J Medicine 2004;23:176-179. [PubMed]
- Legbo JN, Opera WEK and Legbo JK. Sacrococcygeal Teratomas. African Health Sci, Mar 2008;8(1):54-57. [PubMed] [Free Full Text]
- Altman RP, Randolph JG, Lilly JR. Sacrococcygeal teratomas: American academy of pediatrics surgical section survey. J of Pediatric surgery 1974;9:389-98. [PubMed]
- Bilik R, Shandling B, Pope M, et al. Malignant Benign neonatal Sacrococcygeal teratomas. J Pediatric surgery 1993;28:1158-60. [PubMed]
- Sheil AT, Collins KA. Fatal birth trauma due to an undiagnosed abdominal teratomas: Case report and Review of Literature. American J of Forensic medicine and Pathology 2007;28:121-130. [PubMed]
- Bond SJ, Harrison MR, Schnidt KG: Death due to high output cardiac failure in fetal SCT. J pediatric surg 1990;25:1287-91. [PubMed]
- Nakeayama DK, Killian A, Hill LM, et al. The newborn with hydrops and sacrococcygeal teratomas of Pediatric surgery.1991:26:1435-8. [PubMed]
- Roman AS, Monteagudo A, et al, First trimester diagnosis of Sacrococcygeal Teratomas: the role of three dimensional Ultrasound. Obstet Gynecology 2004;23:612-614. [PubMed]
- Keslar PJ, Buck JL. Germ cell tumours of the sacrococcygeal region: radiologic –pathologic correlation: Radiographics 1994;14:607-20: Quiz 625-2. [PubMed]
- Hedrich HL, Flake AW, Crombleholme TM et al. Sacrococcygeal Teratomas: Prenatal assessment, fetal intervention and outcome of Pediatric surgery 2004;39:430-438. [PubMed]
- Finamore PS, Kontopoulos, Price M, et al. Mirror syndrome associated with Sacrococcygeal teratoma case report: J of reproductive medicine 2007;52:225-7. [PubMed]
- Holcroft CJ, Blackmore KJ, Gurewitsch ED, et al. Large Sacrococcygeal teratomas: Could early delivery improve outcome? : Fetal Diagn Ther 2008;24:55-60. [PubMed]
- Hirose S, Farmer DL. Fetal surgery for Sacrococcygeal Teratomas. Clin Perinatol 2003;30:493-506. [PubMed]
- Derix JP, De Backer A, van de Schoot L, et al. Long term functional sequelae of Sacrococcygeal teratomas: a national study in Netherland. J Pediatr Surg 2007;42:1122-6. [PubMed]
- Tsuchida Y, Hasegawa H. The diagnostic values of alpha fetoprotein in infants and children with teratomas: A questionary survey in Japan. J of Pediatric surgery 1983;18:152-55. [PubMed]