Safaa I. Ghaly 1, Marwa A. Khairy 2, Eman A. Mohammed 3, Mohamed M. Kamal 4
Author affiliations:
Background and objective: Intraoperative a single bolus of tranexamic acid may be inadequate to prevent bleeding in the early postoperative period. We compared the effect of two dose regimens of tranexamic acid in reducing perioperative blood loss and the amount of allogeneic blood transfusion in transurethral resection of prostate (TURP).
Methods: A total of 50 patients electively posted for TURP, were randomly assigned to receive either a single bolus dose of tranexamic acid 10 mg/kg (Group A), or a bolus of tranexamic acid 10 mg/kg followed by an infusion of tranexamic acid @1 mg/kg/h till 4 h postoperatively (Group B). Perioperative blood loss and the amount of blood transfused were the primary outcome. Postoperative hemoglobin and hematocrit levels, incidence of deep vein thrombosis and any convulsions were the secondary outcomes.
Results: There was no significant difference among patients in both groups regarding intraoperative and postoperative blood loss at 6 h and 48 h postoperatively. However, the postoperative blood loss at 24 h was significantly higher among patients in Group A (P = 0.014).
Conclusion: Tranexamic acid used as intravenous bolus followed by infusion continued in the postoperative period is more effective in reduction of perioperative blood loss in comparison to single bolus in transurethral resection of prostate.
Abbreviations: TURP: transurethral resection of prostate, TXA: tranexamic acid, Hct: hematocrit, DVT
Key words: Antifibrinolytics, blood loss, transurethral resection of prostate, tranexamic acid.
Citation: Ghaly Tawadros SI, Kamal MM, Mohammed EA, Khairy MA. Single intravenous bolus vs. continuous infusion of tranexamic acid to reduce blood loss in transurethral resection of prostate: a prospective randomized double-blind study. Anaesth. pain intensive care 2025;29(2):210-216. DOI: 0.35975/apic.v29i2.2705
Received: November 10, 2024; Reviewed: February 03, 2025; Accepted: February 05, 2025
Transurethral resection of the prostate (TURP) remains the gold standard treatment of benign prostatic hyperplasia.1,2 Patients undergoing TURP mostly belong to the high-risk group owing to their age and related co-morbidities. Perioperative morbidity from this procedure ranges between 18% and 26% and the mortality rate may be as high as 1 %.3
The surrounding prostate tissue is highly vascularized and can be easily breached during surgery, so perioperative bleeding is considerable.4
Significant blood loss necessitating transfusion during TURP ranges from 0.4% to 7.1%.5 To minimize blood loss, several strategies such as intraoperative estrogen injection and 5-alpha reductase inhibitor have been suggested.6 The release of urokinase from the prostatic tissues, leading to elevated urinary fibrinolytic activity is also believed to contribute to blood loss.7 Therefore, the assessment of the impact of antifibrinolytic agent on reducing hemorrhage during TURP has been studied.8
Tranexamic acid (TXA) is an antifibrinolytic drug which has been extensively studied to reduce blood loss in different procedures. It can bind to plasminogen to inhibit the interaction between plasmin and fibrin, thus preventing the disintegration of the fibrin clot.9 There are different dose recommendations for surgical patients, but higher doses can lead to intravascular thrombosis and seizure like activity.10 Therefore the optimal dosing , timing and root of TXA administration in TURP surgeries need further research.
We aimed to compare the effect of a low dose TXA infusion, following a bolus dose, in reducing perioperative blood loss and the amount of allogeneic blood transfusion in TURP in comparison to a single bolus dose.
This prospective randomized double-blind clinical study was conducted at Ain Shams University Hospitals, Cairo, Egypt. Patients’ enrollment started from September 2019 over a period of 6 months, after approval by the research ethical committee of Faculty of Medicine, Ain Shams University. (No. FMASU MS284 /2019) and the protocol was registered at PAN -African Clinical Trials Registry with No. IDPACTR202407652050074.
The primary outcomes were the perioperative blood loss and need for allogeneic blood transfusion. The secondary outcomes postoperative hemoglobin (Hb), hematocrit (Hct) value and the incidence of deep vein thrombosis (DVT) or convulsions.
We enrolled 50 male patients in the study, 50-70 y old, ASA I or II, scheduled to undergo elective TURP. Patients with a history of bleeding diathesis, pulmonary embolism, DVT, a known allergy to TXA or having any contraindications for spinal anesthesia were excluded. All patients provided informed consent.
Patients were randomly allocated, by computer-generated lists and closed-envelope method, to one of the following groups; Group A (n=25) to receive single bolus of TXA 10 mg/kg 10 min after spinal anesthesia (bolus group) followed by normal saline infusion through syringe pump till 4 h postoperatively. Patients in Group B (n=25) to receive single bolus dose of TXA 10 mg/kg, 10 min after spinal anesthesia followed by infusion of TXA through syringe pump @1 mg/kg/h till 4 h postoperatively (infusion group).
The drug infusions were prepared by a person who was not involved in data collection or patient management preoperatively. Data collection was carried out by a person who was completely blinded to the randomization. The person who analyzed the data was not involved in randomization or data collection.
2.1. Anesthetic technique
Routine preoperative assessment was done, base-line values of Hb and Hct were recorded. A wide bore intravenous (i.v) cannula was inserted and fixed at the dorsum of the hand then 10 mL/kg of lactated Ringer’s solution was administered and maintained at 8 mL/kg/h. Standard monitoring including heart rate (HR), blood pressure (BP) and oxygen saturation (SpO2) was used and baseline values were recorded. In cases of failed spinal anesthesia, general anesthesia was prepared.
Patients were placed in the sitting position and spinal anesthesia was performed under complete aseptic conditions with a 25-gauge Quincke needle with a midline approach, at the L3-L4 or L4-L5 interspaces using hyperbaric bupivacaine. Patients were immediately turned to supine position, while supplemental oxygen was administered at a rate of 4-6 L/min facemask. The temperature of the operating room and post anesthesia care unit (PACU) was kept at 22-26 degree throughout the study. They received single bolus dose of TXA (10mg/kg) slowly i.v 10 minutes after spinal anesthesia. In Group A (bolus group), patients had an infusion syringe of 50 mL 0.9% normal saline with calculated rate as the infusion group till 4 h postoperatively.
In Group B (infusion group) ,infusion syringe was prepared by adding TXA (500 mg/5 mL) to 0.9 % normal saline till reaching 50 mL with infusion rate (1 mg/kg/h) till 4 h postoperatively. Vital parameters were monitored for the rest of the operation and in the PACU. Any episode of hypotension (systolic BP < 90 mm.Hg or >15% drop from the base line BP) was treated with i.v ephedrine (3-6 mg). A decrease in HR (HR<50 beats per minute) was treated with incremental doses of intravenous atropine (0.5 mg).
Demographic data (age, ASA classification) and total duration of surgery in minutes (defined as time from the start of the procedure until the insertion of urinary catheter at the end of the procedure) were recorded.
Intraoperative blood loss was estimated using the formula: Blood loss in mL = (Hb content of the irrigation fluid (gm/L) X Volume (L) x 1000) / (Pre-operative Hb (gm/dl) x5.2)
The volume of irrigation fluid was measured, and samples collected from the bucket after continuous stirring for 5 minutes, were sent for hemoglobin estimation.
A transfusion trigger for blood was loss of more than 20% of total blood volume or hemoglobin concentration less than 8 g/dl, whichever was less. The decision for blood transfusion was made by the same attending consultant anesthesiologist in all cases. Whole blood units or blood products transfused intraoperatively were recorded.
Postoperative blood loss was assessed by collection in the drain at the end of 6 h, 24 h and 48 h. Hb and Hct levels were also recorded 6 h, 24 h and 48 h postoperatively.
Patients were monitored clinically for evidence of DVT twice daily postoperatively till time of discharge, by assessing calf swelling, tenderness, and edema of the leg. Convulsions or seizure like episodes were also monitored.
2.2 Sample Size calculations
Using STATA program, setting alpha error at 5% and power at 90%, result from previous study[11], showed that the mean postoperative blood loss at 24 h, in single dose group was 56.9 ± 16.2 while in infusion group, the mean was 39 ± 12.4. Based on this, the needed sample is 20 cases per group. Taking in consideration a dropout rate of about 12%, we planned to enroll 25 patients in each arm.
2.3 Statistical analysis
Recorded data were analyzed using the statistical package for social sciences, version 20.0 (SPSS Inc., Chicago, Illinois, USA). Quantitative data were expressed as mean ± standard deviation (SD). Qualitative data were expressed as frequency and percentage.
The following tests were done: Independent-samples t-test of significance was used when comparing between two means. Mann Whitney U test: for two-group comparisons in non-parametric data. Chi-square (c2) test of significance was used in order to compare proportions between qualitative parameters. Kaplan-Meier Survival Analysis: is a descriptive procedure for examining the distribution of time-to-event variables. Log rank test to compare time-to-event variables by levels of a factor variable. The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, Probability (P < 0.05) was considered significant, P < 0.001 was considered as highly significant.
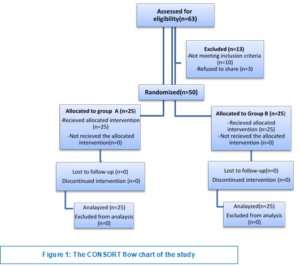
A total of 63 patients were assessed for eligibility.10 patients did not meet the the inclusion criteria and 3 patients refused to participate in the study. Ultimately the study included a total of 50 patients who provided their informed consent. They were randomized into 2 equal groups, Group A and Group B, each consisted of 25 patients (Figure 1).
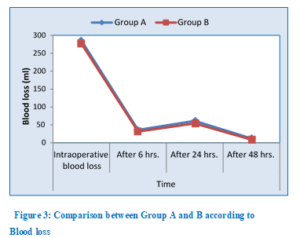
Demographic data (age and ASA classification) and duration of surgery were comparable in the two groups with no significant difference (Table 1). There was no statistically significant difference among patients in both groups regarding intraoperative and postoperative blood loss at 6 h and 48 h. However, the mean values for postoperative blood loss at 24 h was significantly higher among patients in Group A than in Group B (P= 0.014) as shown in Table 2 and Figure 3. No blood transfusion was needed in both groups.
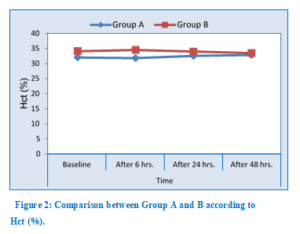
Although non-statistically significant, Hb and Hct values among Group A were lower than Group B at 6, 24 and 48 h postoperatively as shown in Table 3-4 and Figure 2.
There were no recorded cases regarding the incidence of postoperative convulsions or DVT in both groups.
In the current study, TXA reduced blood loss effectively when used as a bolus followed by an infusion in comparison to a single intravenous bolus in patients undergoing TURP.
The prostate has a rich blood supply, and the surrounding tissue contains large venous sinuses.12 When an electric scalpel is used to remove prostate tissue, it squeezes the affected fibrin then dissolves into soluble fibrin degradation products, which cause bleeding.13
venous sinuses, releasing a large number of fibrinolytic enzymes into the blood which activates fibrinolysis. The TXA is a synthetic anti‑fibrinolytic with a chemical structure similar to that of lysine.14 It can restrain the fibrinolytic enzyme adsorption of fibrin and promote blood clot formation; thus, it has hemostatic effects during TURP surgery.
In the current study, TXA was administered immediately after spinal anesthesia in two different ways, as a single bolus dose and as an infusion continued in the early postoperative period after a single bolus dose. There was a significant difference in postoperative bleeding at the end of 24 h with the continuous infusion when compared with the single bolus method (P = 0.014).
In a previous study, it was found that a single bolus dose of TXA of 10 mg/kg produces a therapeutic plasma concentration of 5–10 µg/mL which lasts only for three h The effect of a larger dose (20 mg/kg) lasts for approximately 8 h but carries the potential risk of thrombotic activity posing a greater threat.10 Repeated boluses at regular time intervals may not produce uniform plasma concentrations resulting in unpredictable antifibrinolytic activity.15
The early postoperative period is marked by increased release of tissue plasminogen activators resulting in increased fibrinolysis and is thus, the time when optimum levels of antifibrinolytic activity are required. Therefore, we postulated that a low‑dose continuous infusion extending into the early postoperative period would make a significant difference in the management of hemostasis and our results affirmed this.
Our results were similar to a study conducted by Prasad et al.16 They randomly selected 60 patients scheduled for open abdominal tumor surgery. Group A; was administered single bolus dose of TXA (10 mg/kg), 10 min prior to incision (bolus group) followed by normal saline infusion through syringe pump till 4 h postoperatively. Group B; was administered single bolus dose of tranexamic acid (10 mg/kg), 10 min prior to incision followed by infusion of TXA through syringe pump (1 mg/kg/h) till 4 h postoperatively (infusion group). Group C; was administered single bolus of normal saline followed by normal saline infusion through syringe pump (control group). Both techniques did not significantly affect intraoperative blood loss when compared to the placebo group. However, its use significantly reduced postoperative bleeding causing better preservation of hematological indices.
Meng et al. indicated that a bolus dose of TXA could reduce intraoperative and 4 h postoperative blood loss resulting from TURP surgery, but it had no significant impact on 24 h postoperative blood loss. This was comparable to our results at 24 h postoperatively.17
A meta-analysis involving more than 10,000 patients undergoing different procedures confirmed that the use of TXA resulted in reduction of blood transfusion by 37%.18 In another systematic meta-analysis review, Mina and Garcia, investigated the effectiveness of TXA in the prevention of perioperative bleeding in prostate surgery. They found that TXA is effective at preventing perioperative blood loss compared with the placebo.19 This was in agreement with our findings.
Regarding postoperative complications, none of our patients suffered from convulsions. Several retrospective studies confirmed increased seizures in patients who received TXA with incidence ranging from 0.9 % to 2.9 %.20 Risk factors include higher doses of TXA (more that 45 mg/kg).21 The possible reasons for proconvulsant activity are cerebral vasospasm or cerebral artery thrombosis and inhibition of ɣ amino butyric acid‑A receptors in the brain.22 This was in contrast to our findings as our total dose used was less than that.
There were no recorded cases of postoperative DVT in our study population.
Assessment of perioperative blood loss was subjected to potential variability. We didn’t have facility of thromboelastography which would have been more accurate. Also, we did not apply ultrasonic doppler for early detection of thrombosis and depended upon clinical symptoms and signs, which is not a precise method.
Tranexamic acid is more effective in reducing blood loss in TURP when used as an intravenous bolus followed by an infusion in the postoperative period in comparison to its use as a single intravenous bolus with no apparent complications
7. Availability of data
Data supporting the findings of the current study are available with the corresponding author upon request
8. Competing interests
The authors declare that they have no competing interests.
9. Funding
The authors have no external or industry sources of funding to declare for this study
10. Authors’ contributions
SIG: Data collection.
EAN: Data collection, project administration.
MMK: Study design, data analysis.
MAK: Data analysis, revising paper, supervision.
Author affiliations:
- Safaa Ishak Ghaly Tawadros, MD, Department of Anesthesia, Intensive Care & Pain Management, Faculty of Medicine, Ain Shams University, Cairo, Egypt; Email: sghalymm@hotmail.com ; {ORCID:0000-0003-0967-2463}
- Marwa A. Khairy, MD, Department of Anesthesia, Intensive Care & Pain Management, Faculty of Medicine, Ain Shams University, Cairo, Egypt; Email: marwa.khairy@med.asu.edu.eg
- Eman A. Mohammed, MSc. Department of Anesthesia, Intensive Care & Pain Management, Faculty of Medicine, Ain Shams University, Cairo, Egypt; Email: eman.anabi@gmail.com
- Mohamed M. Kamal, MD, Department of Anesthesia, Intensive Care & Pain Management, Faculty of Medicine, Ain Shams University, Cairo, Egypt; Email: mohamedkamal@med.asu.edu.eg
ABSTRACT
Background and objective: Intraoperative a single bolus of tranexamic acid may be inadequate to prevent bleeding in the early postoperative period. We compared the effect of two dose regimens of tranexamic acid in reducing perioperative blood loss and the amount of allogeneic blood transfusion in transurethral resection of prostate (TURP).
Methods: A total of 50 patients electively posted for TURP, were randomly assigned to receive either a single bolus dose of tranexamic acid 10 mg/kg (Group A), or a bolus of tranexamic acid 10 mg/kg followed by an infusion of tranexamic acid @1 mg/kg/h till 4 h postoperatively (Group B). Perioperative blood loss and the amount of blood transfused were the primary outcome. Postoperative hemoglobin and hematocrit levels, incidence of deep vein thrombosis and any convulsions were the secondary outcomes.
Results: There was no significant difference among patients in both groups regarding intraoperative and postoperative blood loss at 6 h and 48 h postoperatively. However, the postoperative blood loss at 24 h was significantly higher among patients in Group A (P = 0.014).
Conclusion: Tranexamic acid used as intravenous bolus followed by infusion continued in the postoperative period is more effective in reduction of perioperative blood loss in comparison to single bolus in transurethral resection of prostate.
Abbreviations: TURP: transurethral resection of prostate, TXA: tranexamic acid, Hct: hematocrit, DVT
Key words: Antifibrinolytics, blood loss, transurethral resection of prostate, tranexamic acid.
Citation: Ghaly Tawadros SI, Kamal MM, Mohammed EA, Khairy MA. Single intravenous bolus vs. continuous infusion of tranexamic acid to reduce blood loss in transurethral resection of prostate: a prospective randomized double-blind study. Anaesth. pain intensive care 2025;29(2):210-216. DOI: 0.35975/apic.v29i2.2705
Received: November 10, 2024; Reviewed: February 03, 2025; Accepted: February 05, 2025
1. INTRODUCTION
Transurethral resection of the prostate (TURP) remains the gold standard treatment of benign prostatic hyperplasia.1,2 Patients undergoing TURP mostly belong to the high-risk group owing to their age and related co-morbidities. Perioperative morbidity from this procedure ranges between 18% and 26% and the mortality rate may be as high as 1 %.3
The surrounding prostate tissue is highly vascularized and can be easily breached during surgery, so perioperative bleeding is considerable.4
Significant blood loss necessitating transfusion during TURP ranges from 0.4% to 7.1%.5 To minimize blood loss, several strategies such as intraoperative estrogen injection and 5-alpha reductase inhibitor have been suggested.6 The release of urokinase from the prostatic tissues, leading to elevated urinary fibrinolytic activity is also believed to contribute to blood loss.7 Therefore, the assessment of the impact of antifibrinolytic agent on reducing hemorrhage during TURP has been studied.8
Tranexamic acid (TXA) is an antifibrinolytic drug which has been extensively studied to reduce blood loss in different procedures. It can bind to plasminogen to inhibit the interaction between plasmin and fibrin, thus preventing the disintegration of the fibrin clot.9 There are different dose recommendations for surgical patients, but higher doses can lead to intravascular thrombosis and seizure like activity.10 Therefore the optimal dosing , timing and root of TXA administration in TURP surgeries need further research.
We aimed to compare the effect of a low dose TXA infusion, following a bolus dose, in reducing perioperative blood loss and the amount of allogeneic blood transfusion in TURP in comparison to a single bolus dose.
2. METHODOLOGY
This prospective randomized double-blind clinical study was conducted at Ain Shams University Hospitals, Cairo, Egypt. Patients’ enrollment started from September 2019 over a period of 6 months, after approval by the research ethical committee of Faculty of Medicine, Ain Shams University. (No. FMASU MS284 /2019) and the protocol was registered at PAN -African Clinical Trials Registry with No. IDPACTR202407652050074.
The primary outcomes were the perioperative blood loss and need for allogeneic blood transfusion. The secondary outcomes postoperative hemoglobin (Hb), hematocrit (Hct) value and the incidence of deep vein thrombosis (DVT) or convulsions.
We enrolled 50 male patients in the study, 50-70 y old, ASA I or II, scheduled to undergo elective TURP. Patients with a history of bleeding diathesis, pulmonary embolism, DVT, a known allergy to TXA or having any contraindications for spinal anesthesia were excluded. All patients provided informed consent.
Patients were randomly allocated, by computer-generated lists and closed-envelope method, to one of the following groups; Group A (n=25) to receive single bolus of TXA 10 mg/kg 10 min after spinal anesthesia (bolus group) followed by normal saline infusion through syringe pump till 4 h postoperatively. Patients in Group B (n=25) to receive single bolus dose of TXA 10 mg/kg, 10 min after spinal anesthesia followed by infusion of TXA through syringe pump @1 mg/kg/h till 4 h postoperatively (infusion group).
The drug infusions were prepared by a person who was not involved in data collection or patient management preoperatively. Data collection was carried out by a person who was completely blinded to the randomization. The person who analyzed the data was not involved in randomization or data collection.
2.1. Anesthetic technique
Routine preoperative assessment was done, base-line values of Hb and Hct were recorded. A wide bore intravenous (i.v) cannula was inserted and fixed at the dorsum of the hand then 10 mL/kg of lactated Ringer’s solution was administered and maintained at 8 mL/kg/h. Standard monitoring including heart rate (HR), blood pressure (BP) and oxygen saturation (SpO2) was used and baseline values were recorded. In cases of failed spinal anesthesia, general anesthesia was prepared.
Patients were placed in the sitting position and spinal anesthesia was performed under complete aseptic conditions with a 25-gauge Quincke needle with a midline approach, at the L3-L4 or L4-L5 interspaces using hyperbaric bupivacaine. Patients were immediately turned to supine position, while supplemental oxygen was administered at a rate of 4-6 L/min facemask. The temperature of the operating room and post anesthesia care unit (PACU) was kept at 22-26 degree throughout the study. They received single bolus dose of TXA (10mg/kg) slowly i.v 10 minutes after spinal anesthesia. In Group A (bolus group), patients had an infusion syringe of 50 mL 0.9% normal saline with calculated rate as the infusion group till 4 h postoperatively.
In Group B (infusion group) ,infusion syringe was prepared by adding TXA (500 mg/5 mL) to 0.9 % normal saline till reaching 50 mL with infusion rate (1 mg/kg/h) till 4 h postoperatively. Vital parameters were monitored for the rest of the operation and in the PACU. Any episode of hypotension (systolic BP < 90 mm.Hg or >15% drop from the base line BP) was treated with i.v ephedrine (3-6 mg). A decrease in HR (HR<50 beats per minute) was treated with incremental doses of intravenous atropine (0.5 mg).
Demographic data (age, ASA classification) and total duration of surgery in minutes (defined as time from the start of the procedure until the insertion of urinary catheter at the end of the procedure) were recorded.
Intraoperative blood loss was estimated using the formula: Blood loss in mL = (Hb content of the irrigation fluid (gm/L) X Volume (L) x 1000) / (Pre-operative Hb (gm/dl) x5.2)

The volume of irrigation fluid was measured, and samples collected from the bucket after continuous stirring for 5 minutes, were sent for hemoglobin estimation.
A transfusion trigger for blood was loss of more than 20% of total blood volume or hemoglobin concentration less than 8 g/dl, whichever was less. The decision for blood transfusion was made by the same attending consultant anesthesiologist in all cases. Whole blood units or blood products transfused intraoperatively were recorded.
Postoperative blood loss was assessed by collection in the drain at the end of 6 h, 24 h and 48 h. Hb and Hct levels were also recorded 6 h, 24 h and 48 h postoperatively.
Patients were monitored clinically for evidence of DVT twice daily postoperatively till time of discharge, by assessing calf swelling, tenderness, and edema of the leg. Convulsions or seizure like episodes were also monitored.
2.2 Sample Size calculations
Using STATA program, setting alpha error at 5% and power at 90%, result from previous study[11], showed that the mean postoperative blood loss at 24 h, in single dose group was 56.9 ± 16.2 while in infusion group, the mean was 39 ± 12.4. Based on this, the needed sample is 20 cases per group. Taking in consideration a dropout rate of about 12%, we planned to enroll 25 patients in each arm.
2.3 Statistical analysis
Recorded data were analyzed using the statistical package for social sciences, version 20.0 (SPSS Inc., Chicago, Illinois, USA). Quantitative data were expressed as mean ± standard deviation (SD). Qualitative data were expressed as frequency and percentage.
The following tests were done: Independent-samples t-test of significance was used when comparing between two means. Mann Whitney U test: for two-group comparisons in non-parametric data. Chi-square (c2) test of significance was used in order to compare proportions between qualitative parameters. Kaplan-Meier Survival Analysis: is a descriptive procedure for examining the distribution of time-to-event variables. Log rank test to compare time-to-event variables by levels of a factor variable. The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, Probability (P < 0.05) was considered significant, P < 0.001 was considered as highly significant.
3. RESULTS
A total of 63 patients were assessed for eligibility.10 patients did not meet the the inclusion criteria and 3 patients refused to participate in the study. Ultimately the study included a total of 50 patients who provided their informed consent. They were randomized into 2 equal groups, Group A and Group B, each consisted of 25 patients (Figure 1).
Demographic data (age and ASA classification) and duration of surgery were comparable in the two groups with no significant difference (Table 1). There was no statistically significant difference among patients in both groups regarding intraoperative and postoperative blood loss at 6 h and 48 h. However, the mean values for postoperative blood loss at 24 h was significantly higher among patients in Group A than in Group B (P= 0.014) as shown in Table 2 and Figure 3. No blood transfusion was needed in both groups.
Although non-statistically significant, Hb and Hct values among Group A were lower than Group B at 6, 24 and 48 h postoperatively as shown in Table 3-4 and Figure 2.
There were no recorded cases regarding the incidence of postoperative convulsions or DVT in both groups.
4. DISCUSSION
In the current study, TXA reduced blood loss effectively when used as a bolus followed by an infusion in comparison to a single intravenous bolus in patients undergoing TURP.
| Table 1: Comparison between the studied groups according to demographic data. | ||||
| Demographic data | Group A (n=25) |
Group B (n=25) |
t/x2# | P-value |
| Age (y) | 59.40 ± 4.75 | 60.59 ± 4.89 | 0.535 | 0.381 |
| ASA-PS | ||||
| I | 10 (40) | 9 (36) | 0.576 | 0.353 |
| II | 15 (60) | 16 (64) | ||
| Duration of surgery (min) | 51.98 ± 4.16 | 53.01 ± 4.28 | 1.453 | 0.270 |
| Values are presented as mean ± SD or number (%). Group A: Bolus group, Group B: Infusion group.
t-Independent Sample t-test; *P < 0.05 considered significant |
||||
| Table 2: Comparison between the studied groups according the blood loss | ||||
| Blood loss | Group A (n=25) |
Group B (n=25) |
t-test | P-value |
| Intraoperative blood loss | 285.12 ± 65.58 | 276.94 ± 46.22 | 1.361 | 0.291 |
| After 6 h | 35.67 ± 15.11 | 31.25 ± 10.93 | 0.191 | 0.477 |
| After 24 h | 60.96 ± 17.36 | 53.78 ± 13.28 | 2.206 | 0.014* |
| After 48 h | 12.11 ± 10.28 | 8.46 ± 6.86 | 0.519 | 0.338 |
| Values are presented as mean ± SD; Group A: Bolus group, Group B: Infusion group
*P < 0.05 considered significant |
||||
| Table 3: Comparison between the studied groups according to Hb (g/dl) | ||||
| Hb (g/dl) | Group A (n=25) |
Group B (n=25) |
t-test | P-value |
| Baseline | 11.021 ± 1.648 | 12.154 ± 1.854 | 1.006 | 0.247 |
| After 6 h | 10.506 ± 1.339 | 11.124 ± 1.339 | 0.399 | 0.463 |
| After 24 h | 10.815 ± 1.133 | 11.124 ± 1.339 | 1.587 | 0.119 |
| After 48 h | 10.918 ± 0.927 | 11.124 ± 1.236 | 0.144 | 0.656 |
| Values are presented as mean ± SD; Group A: Bolus group, Group B: Infusion group
*P < 0.05 considered significant |
||||
| Table 4: Comparison between the studied groups according to Hct (%) | ||||
| Hct (%) | Group A (n=25) |
Group B (n=25) |
t-test | P-value |
| Baseline | 32.033 ± 4.223 | 34.093 ± 6.489 | 0.147 | 0.653 |
| After 6 h | 31.827 ± 6.489 | 34.505 ± 4.326 | 0.158 | 0.604 |
| After 24 h | 32.548 ± 3.399 | 33.99 ± 4.326 | 0.399 | 0.463 |
| After 48 h | 32.857 ± 2.781 | 33.475 ± 3.811 | 0.071 | 0.752 |
| Data presented as mean ± SD; Group A: Bolus group, Group B: Infusion group
t-Independent Sample t-test; P < 0. (significant) |
||||
The prostate has a rich blood supply, and the surrounding tissue contains large venous sinuses.12 When an electric scalpel is used to remove prostate tissue, it squeezes the affected fibrin then dissolves into soluble fibrin degradation products, which cause bleeding.13


venous sinuses, releasing a large number of fibrinolytic enzymes into the blood which activates fibrinolysis. The TXA is a synthetic anti‑fibrinolytic with a chemical structure similar to that of lysine.14 It can restrain the fibrinolytic enzyme adsorption of fibrin and promote blood clot formation; thus, it has hemostatic effects during TURP surgery.
In the current study, TXA was administered immediately after spinal anesthesia in two different ways, as a single bolus dose and as an infusion continued in the early postoperative period after a single bolus dose. There was a significant difference in postoperative bleeding at the end of 24 h with the continuous infusion when compared with the single bolus method (P = 0.014).
In a previous study, it was found that a single bolus dose of TXA of 10 mg/kg produces a therapeutic plasma concentration of 5–10 µg/mL which lasts only for three h The effect of a larger dose (20 mg/kg) lasts for approximately 8 h but carries the potential risk of thrombotic activity posing a greater threat.10 Repeated boluses at regular time intervals may not produce uniform plasma concentrations resulting in unpredictable antifibrinolytic activity.15
The early postoperative period is marked by increased release of tissue plasminogen activators resulting in increased fibrinolysis and is thus, the time when optimum levels of antifibrinolytic activity are required. Therefore, we postulated that a low‑dose continuous infusion extending into the early postoperative period would make a significant difference in the management of hemostasis and our results affirmed this.
Our results were similar to a study conducted by Prasad et al.16 They randomly selected 60 patients scheduled for open abdominal tumor surgery. Group A; was administered single bolus dose of TXA (10 mg/kg), 10 min prior to incision (bolus group) followed by normal saline infusion through syringe pump till 4 h postoperatively. Group B; was administered single bolus dose of tranexamic acid (10 mg/kg), 10 min prior to incision followed by infusion of TXA through syringe pump (1 mg/kg/h) till 4 h postoperatively (infusion group). Group C; was administered single bolus of normal saline followed by normal saline infusion through syringe pump (control group). Both techniques did not significantly affect intraoperative blood loss when compared to the placebo group. However, its use significantly reduced postoperative bleeding causing better preservation of hematological indices.
Meng et al. indicated that a bolus dose of TXA could reduce intraoperative and 4 h postoperative blood loss resulting from TURP surgery, but it had no significant impact on 24 h postoperative blood loss. This was comparable to our results at 24 h postoperatively.17
A meta-analysis involving more than 10,000 patients undergoing different procedures confirmed that the use of TXA resulted in reduction of blood transfusion by 37%.18 In another systematic meta-analysis review, Mina and Garcia, investigated the effectiveness of TXA in the prevention of perioperative bleeding in prostate surgery. They found that TXA is effective at preventing perioperative blood loss compared with the placebo.19 This was in agreement with our findings.
Regarding postoperative complications, none of our patients suffered from convulsions. Several retrospective studies confirmed increased seizures in patients who received TXA with incidence ranging from 0.9 % to 2.9 %.20 Risk factors include higher doses of TXA (more that 45 mg/kg).21 The possible reasons for proconvulsant activity are cerebral vasospasm or cerebral artery thrombosis and inhibition of ɣ amino butyric acid‑A receptors in the brain.22 This was in contrast to our findings as our total dose used was less than that.
There were no recorded cases of postoperative DVT in our study population.
5. LIMITATIONS
Assessment of perioperative blood loss was subjected to potential variability. We didn’t have facility of thromboelastography which would have been more accurate. Also, we did not apply ultrasonic doppler for early detection of thrombosis and depended upon clinical symptoms and signs, which is not a precise method.
6. CONCLUSION
Tranexamic acid is more effective in reducing blood loss in TURP when used as an intravenous bolus followed by an infusion in the postoperative period in comparison to its use as a single intravenous bolus with no apparent complications
7. Availability of data
Data supporting the findings of the current study are available with the corresponding author upon request
8. Competing interests
The authors declare that they have no competing interests.
9. Funding
The authors have no external or industry sources of funding to declare for this study
10. Authors’ contributions
SIG: Data collection.
EAN: Data collection, project administration.
MMK: Study design, data analysis.
MAK: Data analysis, revising paper, supervision.
11. REFERENCES
- Lokeshwar SD, Harper BT, Webb E, Jordan A, Dykes TA, Neal DE Jr, et al. Epidemiology and treatment modalities for the management of benign prostatic hyperplasia. Transl Androl Urol. 2019;8:529-39. [PubMed] DOI: 21037/tau.2019.10.01
- Franco JV, Jung JH, Imamura M, Borofsky M, Omar MI, Escobar Liquitay CM, et al. Minimally invasive treatments for benign prostatic hyperplasia: A Cochrane network meta-analysis. BJU Int. 2021;7:1-88. [PubMed] DOI: 1111/bju.15653
- Crescenti A, Borghi G, Bignami E, Bertarelli G, Landoni G, Casiraghi GM, et al. Intraoperative use of tranexamic acid to reduce transfusion rate in patients undergoing radical retropubic prostatectomy: Double blind, randomised, placebo-controlled trial. BMJ. 2011;343:d5701. [PubMed] DOI: 1136/bmj.d5701
- Demirel I, Ozer AB, Bayar MK, Erhan OL. TURP syndrome and severe hyponatremia under general anaesthesia. BMJ Case Rep. 2012:2012:bcr-2012-006899. [PubMed] DOI: 1136/bcr-2012-006899
- Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP)--incidence, management, and prevention. Eur Urol. 2006;50(5):969-79. [PubMed] DOI: 1016/j.eururo.2005.12.042
- Zhu YP, Dai B, Zhang HL, Shi GH, Ye DW. Impact of preoperative 5α-reductase inhibitors on perioperative blood loss in patients with benign prostatic hyperplasia: a meta-analysis of randomized controlled trials. BMC Urol. 2015;15:47. [PubMed] DOI: 1186/s12894-015-0043-4
- Samir M, Saafan AM, Afifi RM, Tawfick A. Can high-dose tranexamic acid have a role during transurethral resection of the prostate in large prostates? A randomised controlled trial. Arab J Urol. 2021;20(1):24-9. [PubMed] DOI: 1080/2090598X.2021.1932125
- Ortmann E, Besser MW, Klein AA. Antifibrinolytic agents in current anaesthetic practice. Br J Anaesth. 2013;111(4):549-63. [PubMed] DOI: 1093/bja/aet154
- Hourlier H, Fennema P. Single tranexamic acid dose to reduce perioperative morbidity in primary total hip replacement: A randomised clinical trial. Hip Int. 2013;24(1):63-8. [PubMed] DOI: 5301/hipint.5000090
- Dhir A. Antifibrinolytics in cardiac surgery. Ann Card Anaesth. 2013;16(2):117-25. [PubMed] DOI: 4103/0971-9784.109749
- Kumsar Ş, Dirim A, Toksöz S, Sağlam HS, Adsan Ö. BPH tranexamic acid decreases blood loss during transurethral resection of the prostate (TUR-P). Cent Eur J Urol. 2011;64:156-8. [PubMed] DOI: 5173/ceju.2011.03.art13
- Soliman SA, Wadie BS, Ibrahim EH, Shehab El-Dein AB. Rotoresection versus transurethral resection of the prostate: Short-term evaluation of a prospective randomized study. J Urol. 2007;177(3):1036-9. [PubMed] DOI: 1016/j.juro.2007.01.040
- Porte RJ, Leebeek FW. Pharmacological strategies to decrease transfusion requirements in patients undergoing surgery. Drugs. 2002;62(15):2193-211. [PubMed] DOI: 2165/00003495-200262150-00003
- Dadure C, Sauter M, Bringuier S, Bigorre M, Raux O, Rochette A, et al. Intraoperative tranexamic acid reduces blood transfusion in children undergoing craniosynostosis surgery: a randomized double-blind study. Anesthesiology. 2011;114(4):856-61. [PubMed] DOI: 1097/ALN.0b013e318210f9e3
- Sigaut S, Tremey B, Ouattara A, Couturier R, Taberlet C, Grassin-Delyle S et al. Comparison of two doses of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology. 2014;120(3):590-600. [PubMed] DOI: 1097/ALN.0b013e3182a443e8
- Prasad R, Patki A, Padhy S, Ramchandran G. Single intravenous bolus versus perioperative continuous infusion of tranexamic acid to reduce blood loss in abdominal oncosurgical procedures: A prospective randomized double-blind clinical study. J Anaesthesiol Clin Pharmacol. 2018;34(4):529-34. [PubMed] DOI: 1002/aorn.12743
- Meng QQ, Pan N, Xiong JY, Liu N. Tranexamic acid is beneficial for reducing perioperative blood loss in transurethral resection of the prostate. Exp Ther Med. 2019;17(1):943-7. [PubMed] DOI: 3892/etm.2018.7025
- Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054. [PubMed] DOI: 1136/bmj.e3054
- Mina SH, Garcia-Perdomo HA. Effectiveness of tranexamic acid for decreasing bleeding in prostate surgery: a systematic review and meta-analysis. Cent Eur J Urol. 2018;71(1):72-7. [PubMed] DOI: 5173/ceju.2017.1581
- Sharma V, Katznelson R, Jerath A, Garrido-Olivares L, Carroll J, Rao V, et al. The association between tranexamic acid and convulsive seizures after cardiac surgery: A multivariate analysis in 11,529 patients. Anaesthesia. 2013;69(2):124-30. [PubMed] DOI: 1111/anae.12516
- Sander M, Spies CD, Martiny V, Rosenthal C, Wernecke KD, von Heymann C. Mortality associated with administration of high-dose tranexamic acid and aprotinin in primary open-heart procedures: a retrospective analysis. Crit Care. 2010;14(4):R148. [PubMed] DOI: 1186/cc9216
- Lecker I, Wang DS, Whissell PD, Avramescu S, Mazer CD, Orser BA. Tranexamic acid-associated seizures: Causes and treatment. Ann Neurol. 2016;79(1):18-26. [PubMed] DOI: 10.1002/ana.24558