Tayyaba Tahira 1, Ahmad Zahid Rao 2, Farah Naz 3, Anum Hasan 4, Muhammad Mubashir 5, Zahid Akhtar Rao 6
Author affiliations:
Background: Despite the growing interest in Propolis as an endodontic irrigant, limited research has compared its clinical efficacy and post-operative outcomes with Sodium Hypochlorite (NaOCl), especially concerning post-operative pain. The aim of this study was to evaluate and compare the analgesic properties of 5.25% NaOCl and 20% Propolis, during the initial phase of multi visit endodontic treatment in patients with symptomatic irreversible pulpitis (SIP).
Methodology: A double blind randomized controlled trial was conducted on 44 teeth of patients presenting with SIP in premolars. The patients were allocated to two groups receiving different endodontic irrigants, Group A: NaOCl, and Group B: Propolis solution. These were used in equal volumes during the chemo-mechanical process, on the first visit of treatment. The pain scores were recorded using the visual analog pain scale, preoperatively and postoperatively at 24, 48 and 72 hours. The number of postoperative rescue medicine used by patients was recorded. Data were analyzed using Chi-square test, two-sample t-tests, and two-way repeated measures ANOVA, with Bonferroni post hoc analysis.
Results: The results revealed a significant decrease in postoperative pain over time across both the groups (p = 0.000). Group A and B exhibited a comparable reduction in pain scores from baseline to post-intervention (P = 0.139). Group B required significantly less rescue analgesia within the first 24 hours (P = 0.014).
Conclusions: Propolis and NaOCl are equally effective endodontic irrigants in reducing pain intensity after endodontic treatment of premolars with SIP. Propolis has better pain control during the initial postoperative period.
Trial registration: Retrospectively registered at ClinicalTrials.gov (NCT05974748).
Abbreviations: NaOCl: Sodium Hypochlorite, SIP: symptomatic irreversible pulpitis, VAS: visual analog scale
Keywords: analogue pain scale, sodium hypochlorite, propolis, pulpitis, root canal irrigant.
Citation: Tahira T, Rao AZ, Naz F, Hasan A, Mubashir M, Rao ZA. Comparison of analgesic properties of propolis with sodium hypochlorite as an endodontic irrigant: a randomized controlled trial. Anaesth. pain intensive care 2025;29(1):139-147.
DOI: 10.35975/apic.v29i1.2665
Received: September 11, 2024; Reviewed: November 08, 2024; Accepted: December 15, 2024
Symptomatic irreversible pulpitis (SIP) is a common endodontic condition characterized by severe, spontaneous, lingering dental pain, often necessitating root canal treatment to alleviate the symptoms.1 Successful root canal therapy depends on effective disinfection of the root canal system, which can be achieved through mechanical instrumentation and chemical irrigation. Post treatment pain can occur after initiation of endodontics and is dependent on factors such as severity of pre-operative pain and physical and chemical irritation to the periapical tissues.2 Several approaches have been used to manage pre and postoperative pain of SIP, including non-narcotic analgesics3,4, narcotic analgesics,5 different endodontic irrigating solutions.6
Sodium hypochlorite (NaOCl) has long been considered the gold standard endodontic irrigant due to its strong antimicrobial properties and tissue-dissolving abilities.7 However, concerns regarding its cytotoxicity and tendency to irritate the periapical tissues, even at low concentrations particularly when it extends beyond the apex, have prompted the search for alternative, biocompatible and equally effective irrigants. One alternative approach is to use Propolis as an endodontic irrigant that has negligible secondary effects.
Propolis,8 a natural resinous substance collected by honeybees from plants, has shown promising anti-inflammatory properties under both in vitro and in vivo conditions by inhibiting the production of prostaglandins and leukotrienes.9 One of the main components of honeybee Propolis, caffeine phenethyl ester, which is derived from hives, is anti-inflammatory. This natural material has also found its way into the field of dentistry, where it has been utilized in various dental procedures such as alleviation of dentine hypersensitivity10 and periodontitis.11 Several studies have investigated the use of Propolis as an endodontic irrigant and reported encouraging results, suggesting its potential as a safe and effective alternative to NaOCl.12–14
Despite the growing interest in Propolis as an endodontic irrigant, limited research has compared its clinical efficacy and post-operative outcomes with NaOCl, especially concerning post-operative pain.15 This study aimed to conduct a comparative evaluation of the analgesic properties of 5.25% NaOCl and 20% Propolis, during the initial phase of multi visit endodontic treatment in patients with symptomatic irreversible pulpitis (SIP). By analyzing the impact of these two irrigants on pain perception, the study intends to provide valuable insights into their respective roles in endodontic therapy and investigate some competent herbal alternatives for this important step in endodontics.
This randomized controlled trial was prepared according to the CONSORT 2010 Guidelines.
2.1. Preparation of Propolis Hydroalcoholic solution
The process began by obtaining Propolis extract powder from a reputable company, The Propolis powder was mixed with 100% ethanol in a hermetically sealed glass beaker to create the solution at a pharmaceutical laboratory. The proportion used was 1 g of Propolis powder to 3 mL of ethanol. The beaker was then placed in a dark environment and left to incubate for a duration of one week, during which time it was continuously agitated. After the week had passed, the ethanol solution was subjected to centrifugation at a speed of 1000 revolutions per minute. This process resulted in the separation of the supernatants, which were then collected and filtered using Whatman #4 filter paper. The resulting extract was subsequently dissolved in a 10% hydroalcoholic solution, which is a combination of ethanol and water. The proportion used for this step was 20 mL of Propolis extract per 100 mL of 10% ethanol solution. Finally, the solution was stored in an opaque vessel that was hermetically sealed, and it was kept at room temperature.
2.2. Sample size calculation
The sample size was based on the numbers included in several studies investigating outcome of Propolis as an endodontic irrigant,16 as well as outcome studies investigating post-operative pain between different endodontic irrigants and 5.25% NaOCl as control.17 The aim of this study is to investigate the post-operative pain control with Propolis and NaOCl, so a sample size was calculated using OpenEpi (version 3.01 developed by Emory University, Atlanta, GA) sample size calculator using power 80%, significance 95%, percentage of Exposed with Outcome 50%. Total sample size was determined to be 38. Consequently, 19 patients were required in each group, exposed and non-exposed group. This number was increased to 44 to make up for drop-outs during follow-up. However, there were none lost to follow-up (Figure 1).
2.3. Participants and eligibility criteria
The patients were randomly assigned to either the Propolis group or the NaOCl group using the sealed envelope technique. The selection of patients was based on their endodontic diagnosis of symptomatic irreversible pulpitis (SIP).
To ensure the accuracy of the findings, only patients aged 18 to 60 years, with good systemic health were included in the study. The patients had to have SIP with normal periapex, or symptomatic apical periodontitis affected maxillary or mandibular premolars, and their preoperative pain scores had to fall within the moderate to severe range (4-10) on a visual analogue scale (VAS).
The study excluded ASA 3 and above individuals, severely damaged teeth, calcified canals, root resorption, previously root canal treated teeth, pregnant or lactating women, and individuals who had allergies to the bee products.
2.3. Trial design and endodontic procedure
The study was designed as a double blind randomized controlled trial with two-arms parallel group and 1:1 allocation. This trial is retrospectively registered at ClinicalTrials.gov Identifier: NCT05974748. This study was conducted in the Department of Operative Dentistry and Endodontics. Ethical clearance was obtained from the Institutional Review Board (IRB-2908/DUHS/Approval/2023/113) in March 2023. The recruitment of patients was done within six months from IRB approval i.e. from March 15, 2023 to September 23, 2023. Patients or the public were not involved in the design, conduct, or reporting, or dissemination plans of our research. The data collection was stopped once the sample size of 44 was achieved. The study was performed in accordance with the guidelines provided by the Declaration of Helsinki. Each patient was provided with a detailed explanation of the study's objective and their written informed consent was obtained.

An intern posted on rotation duty carried out the procedure under supervision of the principal investigator. Prior to treatment, the pulp vitality was confirmed using Endo Ice (Coltene Endo Ice). Following radiographic examination, adequate anesthesia was achieved by 1.8% lidocaine (Medicaine), after rubber dam isolation, an access cavity preparation was carried out. Using periapical digital radiographs (Digora; Soredex) and an electronic apex locator (Root ZX II Dentsply Sirona), the working lengths to apical constriction were confirmed. K-files # 8-#25 (Mani Japan) were used to perform the pulpectomy up to the respective working lengths of the teeth under study.
2.4. Randomization and blinding
It was a double-blind study. Patients, and outcome assessor were blinded to the groups. The operator could not be blinded because of the difference in the color of endodontic irrigants.
The participants were randomly divided into two groups using the sealed envelope method. Sealed envelopes were prepared by co-investigators containing 22 ‘Group A’ and 22 ‘Group B’ assignments. These were then shuffled and placed in a box. Patients were allowed to pick up the sealed envelopes and hand it over to the intern on duty. In Group A (Control group), 3 mL of 5.25% NaOCl was used to irrigate each canal between each instrumentation. In Group B (Intervention group), 3 mL of 20% hydroalcoholic Propolis solution was used to irrigate each canal, between each instrument (Figure 2). A 31-G double side-vented needle, (NaviTip; Ultradent, South Jordan, UT) was used in a continuous up and down motion within 2 mm of the working length.
A dry, cotton pellet was inserted into the access cavities, and 3M Cavit Temporary Filling Material was placed to seal the access. Proforma and pain intensity scale were provided to the participants, to self-record their pain at 24, 48, and 72 hours following the procedure. Patients were advised to take rescue medicine (ibuprofen 400 mg (Brufen, Abbott Laboratories (Pakistan) Ltd) if moderate to severe pain develop. At the beginning of the second appointment (3 days later), the proforma was collected from the patients, changes in post-operative pain scores were assessed by an intern on duty (not on research). The tooth was isolated with rubber dam, temporary dressing was removed, canals irrigated with saline to remove any remnants of previous irrigating solution. Endodontic treatment was then completed under standard protocols.
2.5. Outcomes
The primary outcome measure of this trial was to evaluate the change in post-operative pain intensity on visual analog scale (VAS). The patient self-recorded the VAS, which was then assessed by an intern on a scale of 0-10 with 0 being no pain, 10 being worst pain. The VAS scores were recorded once in pre-operative condition and thrice in post-operative condition at 24 hours, 48 hours, and 72 hours. The secondary outcome measure was to assess the need of rescue medicine in both groups in post-operative conditions. This was self-recorded by the patient within the time frame of first 24 hours post-operatively.
2.6. Statistical analysis
Statistical analyses were performed using SPSS 24 software for Windows (SPSS Inc., Chicago, IL., USA). The means and standard deviations were calculated for patient demographics, pain intensity scores (VAS from 0 to 10), and rescue medicine intake. The patient demographics were assessed for a significant difference between both groups, using the chi-square test for the gender distribution and the two-sample t-test for the mean age of patients. Moreover, the preoperative VAS score and the recue medicine intake were also assessed using the two-sample t-test between both groups. A two-way repeated measure analysis of variance (ANOVA) with one within-subjects factor (time) and one between-subjects factor (group) was applied to examine changes in the outcomes (VAS scores) based on the main effects, and a two-way interaction effect. The post hoc Bonferroni correction was performed to control the overall Type I error rate for multiple comparisons. Partial eta squared (ƞp2) effect sizes and observed power were reported for ANOVA. The level of significance was set at 0.05 for all tests.
3.1. Patient demographics
The patients’ return rate was 100% in both groups. The mean age of patients was 34.68 ± 9.71 years and 75.0% of them were female whereas 25.0% were male. As shown in Table 1, the patients demographics in terms of gender (P = 0.72, chi square test), the mean age of patients (P = 0.73, two sample t-test), and their VAS pain scores before the treatment (P = 0.37, two sample t test), were not significantly different between the study groups.
3.2. Pain scores
Figure 3 shows the trend of VAS pain scores with respect to time in both groups. The pain decreased immediately after treatment in both groups with a more rapid decrease in Group B at 48 hr. Table 2 gives the means and standard deviations of the scores at the different time in both groups.
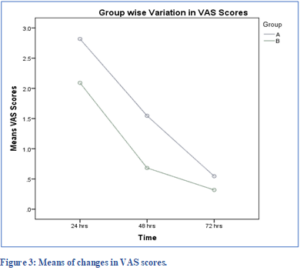
Table 3 shows the results from the two-way repeated measures ANOVA. The main effect of time on the average VAS scores was found to be statistically significant, F (2,84) = 32.710, P < 0.001, ƞp2=0.438. However, the main effect of treatment group on the average VAS score across time F (1,42) =2.274, P = 0.139, ƞp2 = 0.051, and the interaction effect of Time x Group F (2,84) = 0.867, P = 0.424, ƞp2 = 0.020 were not statistically significant.
The significant main effect of time was further analyzed using the Bonferroni adjusted pairwise comparison for time intervals. Table 4 shows that all pairwise comparisons on the average VAS scores were statistically significant.
3.3. Rescue medicine
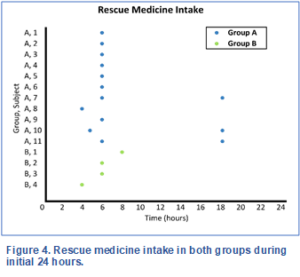
Figure 4 shows the rescue medicine intake in both groups during the initial 24 hours post treatment. In Group A, 11 out of 22 participants needed rescue medicine at 4, 5 or 6 hourly intervals. Among these 11, there were 3 who needed another rescue medicine at 18 hours. In Group B, only 4 out of 22 participants needed a single rescue medicine at 4, 6 or 8 hourly intervals, indicating that Group B may have had better pain control during this initial postoperative period.
A two-sample t-test was conducted to analyze the difference in rescue medicine intake between the two groups. The results indicate a significant difference (t(42) = 2.578, P = 0.014) between Group A (M = 0.64, SD = 0.727) and Group B (M = 0.18, SD = 0.395). Group B, on average, took 0.46 rescue medicine less than Group A. The 95% confidence interval of the difference between the means was (0.099, 0.810).
A two-sample t-test was conducted to analyze the difference in rescue medicine intake between the two groups. The results indicate a significant difference (t(42) = 2.578, P = 0.014) between Group A (M = 0.64, SD = 0.727) and Group B (M = 0.18, SD = 0.395). Group B, on average, took 0.46 rescue medicine less than Group A. The 95% confidence interval of the difference between the means was (0.099, 0.810).
This study is one of the few research projects18 which compared the effects of NaOCl and Propolis solution as endodontic irrigants on post-operative pain.
Our study aimed to compare the effects of 5.25% sodium hypochlorite (NaOCl) and 20% Propolis solution as endodontic irrigants on post-operative pain in patients with SIP. The findings from this study offer valuable insights into the selection of irrigants and their impact on patient comfort and postoperative pain management.
In the present study, only patients with good systemic health ASA-I and ASA-2 were included, so that the impact of systemic factors on root canal treatment’s outcome and healing process could be excluded as much as possible. And so that any unexpected adverse reactions could be avoided.19
Only premolar teeth with SIP accompanied by clinical symptoms of moderate to severe preoperative pain and with or without tenderness to pressure without apical radiolucency were included. These criteria were specifically chosen for standardization and as these clinical presentations were significant predictors of postoperative pain.19
This study was conducted only in the initial phase of endodontic treatment, so that all patients must receive standard endodontic treatment. Furthermore, it also improved the compliance of patients to follow-up.
Pain was assessed up to 72 h postoperatively because the incidence and severity of post-endodontic pain has been shown to be highest in the first 24 h and decreases substantially to minimal levels.20
Our findings underscore a notable decline in postoperative pain over time for both the NaOCl and Propolis treatment cohorts. This underscores the efficacy of both irrigants in alleviating post-treatment discomfort in patients with SIP, marking a significant positive outcome. Mitigating post-operative pain stands as a pivotal objective in endodontic procedures, elevating the overall patient experience. Similarly, several other studies have also reported positive outcomes with Propolis. Our findings validated those of another study,.18 that compared the effects of propolis nanoparticles and NaOCl as a root canal irrigant on postoperative pain and bacterial reduction in mandibular premolars with necrotic pulps.
Notably, Propolis irrigation has exhibited efficacy in eradicating microbial organisms.21,22 Studies have highlighted Propolis's superiority over calcium hydroxide in combating endodontic infections when used as an intracanal medication,14,23 and in reducing polymicrobial infections in necrotic primary molar root canals.24
The consistent reduction in pain scores observed across both groups at 24-, 48-, and 72-hours post-treatment suggests that Propolis presents itself as a viable substitute for NaOCl as an endodontic irrigant. This discovery holds significant implications, offering clinicians an alternative solution for irrigation, particularly beneficial for patients prone to allergies or sensitivities to NaOCl.25 The growing interest in Propolis within endodontics stems from its documented antimicrobial,24 and anti-inflammatory properties. Notably, it inhibits prostaglandin and leukotriene synthesis, resulting in reduced tissue irritation, attributed in part to the presence of caffeine phenethyl ester.15 Studies employing Propolis have demonstrated its superiority, surpassing even a triple antibiotic blend in combatting microbial organisms like E. faecalis.26 Similarly, our findings lend support to the consideration of Propolis as a promising option in this regard. However, it should be noted that a low concentration of Propolis might limit its efficacy against microbial infection.16
Analgesic administration within the initial 24 hours following SIP treatment remains crucial in addressing patient discomfort.27 Yet, controversies persist regarding the optimal approach to managing post-treatment pain.28 In our investigation, the Propolis group required significantly less rescue analgesics than the NaOCl group. This finding suggests that Propolis may contribute to a more comfortable postoperative experience for patients. The reduced need for rescue medication not only improves patient satisfaction but can also have economic implications by reducing the cost of additional pain management measures.
The strengths of the present study are randomization of both intervention and control groups. This randomized controlled trial demonstrated high internal validity due to its rigorous randomization process, standardized measurement tools, and efforts to control confounding variables such as age, gender, and baseline health status. Secondly, patients and assessors were blinded to the root canal irrigant used, minimizing bias, and ensuring the validity of the study results. Thirdly, the compliance of the patient, there were none lost to follow-up as the study was carried out only in the initial phase of multi visit endodontic treatment.
Although our study yields promising findings, it is imperative to recognize its limitations. One notable challenge arose from occasionally compromised visibility within the irrigating field during the irrigation process. The Propolis irrigant had a cloudy sandy appearance, which made it difficult for operators to fully visualize the treatment area. Additionally, instances occurred where the working length required adjustment during the second visit of root canal treatment due to blockages caused by certain components of the Propolis irrigant. Exploring alternative formulations and concentrations of Propolis in the form of clear solutions may alleviate visibility issues during irrigation, thereby potentially enhancing treatment outcomes.
In conclusion, our study demonstrates that both 5.25% NaOCl and 20% Propolis solution are effective in reducing post-operative pain in patients with SIP. The choice between these two irrigants should consider factors such as patient sensitivities, allergies, and the need for rescue analgesics, where Propolis shows an advantage. These results contribute to the broader discussion on improving patient comfort in endodontic treatments and suggest that Propolis is a promising alternative to traditional NaOCl irrigation. Further research and clinical trials are warranted to explore the applicability of propolis as irrigant in different clinical scenarios.
7. Acknowledgements
The authors would like to thank the pharmaceutical lab for their invaluable input and support throughout the making of Propolis irrigant and the interns posted at the department for their assistance in data collection.
8. Data availability
The numerical data generated in this study is available with the corresponding author and can be requested.
9. Source of funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
10. Conflict of interest statement
The authors have no conflicts of interest to declare.
11. Authors’ contribution
TT: Contributed to conception, design, data acquisition and interpretation, drafted and critically revised the manuscript.
AZR, ZAR: Performed all statistical analyses, drafted, and critically revised the manuscript.
FN: Contributed to concept, design, and critically revised the manuscript.
AH, MM: Contributed to data acquisition and drafted the manuscript.
All authors gave their final approval and agreed to be accountable for all aspects of the work.
Author affiliations:
- Tayyaba Tahira, Operative Dentistry & Endodontics Department, Dow International Dental College, Dow University of Health Sciences, Karachi, Pakistan; Email: tayyaba.tahira@duhs.edu.pk
- Ahmad Zahid Rao, Department of Biomedical Engineering, NED University of Engineering and Technology, Karachi, Pakistan; Email: ahmadrao@neduet.edu.pk
- Farah Naz, Operative Dentistry & Endodontics Department, Dow International Dental College, Dow University of Health Sciences, Karachi, Pakistan; Email: farah.naz@duhs.edu.pk
- Anum Hasan, Operative Dentistry & Endodontics Department, Dow International Dental College, Dow University of Health Sciences, Karachi, Pakistan; Email: anne.sheikh21@gmail.com
- Muhammad Mubashir, Operative Dentistry & Endodontics Department, Dow International Dental College, Dow University of Health Sciences, Karachi, Pakistan; Email: mubashirkh888@gmail.com
- Zahid Akhtar Rao, Department of Anesthesiology & Critical Care, Fazaia Ruth Pfau Medical College, Karachi, Pakistan; Email: zahidrao57@hotmail.com
ABSTRACT
Background: Despite the growing interest in Propolis as an endodontic irrigant, limited research has compared its clinical efficacy and post-operative outcomes with Sodium Hypochlorite (NaOCl), especially concerning post-operative pain. The aim of this study was to evaluate and compare the analgesic properties of 5.25% NaOCl and 20% Propolis, during the initial phase of multi visit endodontic treatment in patients with symptomatic irreversible pulpitis (SIP).
Methodology: A double blind randomized controlled trial was conducted on 44 teeth of patients presenting with SIP in premolars. The patients were allocated to two groups receiving different endodontic irrigants, Group A: NaOCl, and Group B: Propolis solution. These were used in equal volumes during the chemo-mechanical process, on the first visit of treatment. The pain scores were recorded using the visual analog pain scale, preoperatively and postoperatively at 24, 48 and 72 hours. The number of postoperative rescue medicine used by patients was recorded. Data were analyzed using Chi-square test, two-sample t-tests, and two-way repeated measures ANOVA, with Bonferroni post hoc analysis.
Results: The results revealed a significant decrease in postoperative pain over time across both the groups (p = 0.000). Group A and B exhibited a comparable reduction in pain scores from baseline to post-intervention (P = 0.139). Group B required significantly less rescue analgesia within the first 24 hours (P = 0.014).
Conclusions: Propolis and NaOCl are equally effective endodontic irrigants in reducing pain intensity after endodontic treatment of premolars with SIP. Propolis has better pain control during the initial postoperative period.
Trial registration: Retrospectively registered at ClinicalTrials.gov (NCT05974748).
Abbreviations: NaOCl: Sodium Hypochlorite, SIP: symptomatic irreversible pulpitis, VAS: visual analog scale
Keywords: analogue pain scale, sodium hypochlorite, propolis, pulpitis, root canal irrigant.
Citation: Tahira T, Rao AZ, Naz F, Hasan A, Mubashir M, Rao ZA. Comparison of analgesic properties of propolis with sodium hypochlorite as an endodontic irrigant: a randomized controlled trial. Anaesth. pain intensive care 2025;29(1):139-147.
DOI: 10.35975/apic.v29i1.2665
Received: September 11, 2024; Reviewed: November 08, 2024; Accepted: December 15, 2024
1. INTRODUCTION
Symptomatic irreversible pulpitis (SIP) is a common endodontic condition characterized by severe, spontaneous, lingering dental pain, often necessitating root canal treatment to alleviate the symptoms.1 Successful root canal therapy depends on effective disinfection of the root canal system, which can be achieved through mechanical instrumentation and chemical irrigation. Post treatment pain can occur after initiation of endodontics and is dependent on factors such as severity of pre-operative pain and physical and chemical irritation to the periapical tissues.2 Several approaches have been used to manage pre and postoperative pain of SIP, including non-narcotic analgesics3,4, narcotic analgesics,5 different endodontic irrigating solutions.6
Sodium hypochlorite (NaOCl) has long been considered the gold standard endodontic irrigant due to its strong antimicrobial properties and tissue-dissolving abilities.7 However, concerns regarding its cytotoxicity and tendency to irritate the periapical tissues, even at low concentrations particularly when it extends beyond the apex, have prompted the search for alternative, biocompatible and equally effective irrigants. One alternative approach is to use Propolis as an endodontic irrigant that has negligible secondary effects.
Propolis,8 a natural resinous substance collected by honeybees from plants, has shown promising anti-inflammatory properties under both in vitro and in vivo conditions by inhibiting the production of prostaglandins and leukotrienes.9 One of the main components of honeybee Propolis, caffeine phenethyl ester, which is derived from hives, is anti-inflammatory. This natural material has also found its way into the field of dentistry, where it has been utilized in various dental procedures such as alleviation of dentine hypersensitivity10 and periodontitis.11 Several studies have investigated the use of Propolis as an endodontic irrigant and reported encouraging results, suggesting its potential as a safe and effective alternative to NaOCl.12–14
Despite the growing interest in Propolis as an endodontic irrigant, limited research has compared its clinical efficacy and post-operative outcomes with NaOCl, especially concerning post-operative pain.15 This study aimed to conduct a comparative evaluation of the analgesic properties of 5.25% NaOCl and 20% Propolis, during the initial phase of multi visit endodontic treatment in patients with symptomatic irreversible pulpitis (SIP). By analyzing the impact of these two irrigants on pain perception, the study intends to provide valuable insights into their respective roles in endodontic therapy and investigate some competent herbal alternatives for this important step in endodontics.
2. METHODOLOGY
This randomized controlled trial was prepared according to the CONSORT 2010 Guidelines.
2.1. Preparation of Propolis Hydroalcoholic solution
The process began by obtaining Propolis extract powder from a reputable company, The Propolis powder was mixed with 100% ethanol in a hermetically sealed glass beaker to create the solution at a pharmaceutical laboratory. The proportion used was 1 g of Propolis powder to 3 mL of ethanol. The beaker was then placed in a dark environment and left to incubate for a duration of one week, during which time it was continuously agitated. After the week had passed, the ethanol solution was subjected to centrifugation at a speed of 1000 revolutions per minute. This process resulted in the separation of the supernatants, which were then collected and filtered using Whatman #4 filter paper. The resulting extract was subsequently dissolved in a 10% hydroalcoholic solution, which is a combination of ethanol and water. The proportion used for this step was 20 mL of Propolis extract per 100 mL of 10% ethanol solution. Finally, the solution was stored in an opaque vessel that was hermetically sealed, and it was kept at room temperature.
2.2. Sample size calculation
The sample size was based on the numbers included in several studies investigating outcome of Propolis as an endodontic irrigant,16 as well as outcome studies investigating post-operative pain between different endodontic irrigants and 5.25% NaOCl as control.17 The aim of this study is to investigate the post-operative pain control with Propolis and NaOCl, so a sample size was calculated using OpenEpi (version 3.01 developed by Emory University, Atlanta, GA) sample size calculator using power 80%, significance 95%, percentage of Exposed with Outcome 50%. Total sample size was determined to be 38. Consequently, 19 patients were required in each group, exposed and non-exposed group. This number was increased to 44 to make up for drop-outs during follow-up. However, there were none lost to follow-up (Figure 1).
2.3. Participants and eligibility criteria
The patients were randomly assigned to either the Propolis group or the NaOCl group using the sealed envelope technique. The selection of patients was based on their endodontic diagnosis of symptomatic irreversible pulpitis (SIP).
To ensure the accuracy of the findings, only patients aged 18 to 60 years, with good systemic health were included in the study. The patients had to have SIP with normal periapex, or symptomatic apical periodontitis affected maxillary or mandibular premolars, and their preoperative pain scores had to fall within the moderate to severe range (4-10) on a visual analogue scale (VAS).
The study excluded ASA 3 and above individuals, severely damaged teeth, calcified canals, root resorption, previously root canal treated teeth, pregnant or lactating women, and individuals who had allergies to the bee products.
2.3. Trial design and endodontic procedure
The study was designed as a double blind randomized controlled trial with two-arms parallel group and 1:1 allocation. This trial is retrospectively registered at ClinicalTrials.gov Identifier: NCT05974748. This study was conducted in the Department of Operative Dentistry and Endodontics. Ethical clearance was obtained from the Institutional Review Board (IRB-2908/DUHS/Approval/2023/113) in March 2023. The recruitment of patients was done within six months from IRB approval i.e. from March 15, 2023 to September 23, 2023. Patients or the public were not involved in the design, conduct, or reporting, or dissemination plans of our research. The data collection was stopped once the sample size of 44 was achieved. The study was performed in accordance with the guidelines provided by the Declaration of Helsinki. Each patient was provided with a detailed explanation of the study's objective and their written informed consent was obtained.

An intern posted on rotation duty carried out the procedure under supervision of the principal investigator. Prior to treatment, the pulp vitality was confirmed using Endo Ice (Coltene Endo Ice). Following radiographic examination, adequate anesthesia was achieved by 1.8% lidocaine (Medicaine), after rubber dam isolation, an access cavity preparation was carried out. Using periapical digital radiographs (Digora; Soredex) and an electronic apex locator (Root ZX II Dentsply Sirona), the working lengths to apical constriction were confirmed. K-files # 8-#25 (Mani Japan) were used to perform the pulpectomy up to the respective working lengths of the teeth under study.
2.4. Randomization and blinding
It was a double-blind study. Patients, and outcome assessor were blinded to the groups. The operator could not be blinded because of the difference in the color of endodontic irrigants.
The participants were randomly divided into two groups using the sealed envelope method. Sealed envelopes were prepared by co-investigators containing 22 ‘Group A’ and 22 ‘Group B’ assignments. These were then shuffled and placed in a box. Patients were allowed to pick up the sealed envelopes and hand it over to the intern on duty. In Group A (Control group), 3 mL of 5.25% NaOCl was used to irrigate each canal between each instrumentation. In Group B (Intervention group), 3 mL of 20% hydroalcoholic Propolis solution was used to irrigate each canal, between each instrument (Figure 2). A 31-G double side-vented needle, (NaviTip; Ultradent, South Jordan, UT) was used in a continuous up and down motion within 2 mm of the working length.

A dry, cotton pellet was inserted into the access cavities, and 3M Cavit Temporary Filling Material was placed to seal the access. Proforma and pain intensity scale were provided to the participants, to self-record their pain at 24, 48, and 72 hours following the procedure. Patients were advised to take rescue medicine (ibuprofen 400 mg (Brufen, Abbott Laboratories (Pakistan) Ltd) if moderate to severe pain develop. At the beginning of the second appointment (3 days later), the proforma was collected from the patients, changes in post-operative pain scores were assessed by an intern on duty (not on research). The tooth was isolated with rubber dam, temporary dressing was removed, canals irrigated with saline to remove any remnants of previous irrigating solution. Endodontic treatment was then completed under standard protocols.
2.5. Outcomes
The primary outcome measure of this trial was to evaluate the change in post-operative pain intensity on visual analog scale (VAS). The patient self-recorded the VAS, which was then assessed by an intern on a scale of 0-10 with 0 being no pain, 10 being worst pain. The VAS scores were recorded once in pre-operative condition and thrice in post-operative condition at 24 hours, 48 hours, and 72 hours. The secondary outcome measure was to assess the need of rescue medicine in both groups in post-operative conditions. This was self-recorded by the patient within the time frame of first 24 hours post-operatively.
2.6. Statistical analysis
Statistical analyses were performed using SPSS 24 software for Windows (SPSS Inc., Chicago, IL., USA). The means and standard deviations were calculated for patient demographics, pain intensity scores (VAS from 0 to 10), and rescue medicine intake. The patient demographics were assessed for a significant difference between both groups, using the chi-square test for the gender distribution and the two-sample t-test for the mean age of patients. Moreover, the preoperative VAS score and the recue medicine intake were also assessed using the two-sample t-test between both groups. A two-way repeated measure analysis of variance (ANOVA) with one within-subjects factor (time) and one between-subjects factor (group) was applied to examine changes in the outcomes (VAS scores) based on the main effects, and a two-way interaction effect. The post hoc Bonferroni correction was performed to control the overall Type I error rate for multiple comparisons. Partial eta squared (ƞp2) effect sizes and observed power were reported for ANOVA. The level of significance was set at 0.05 for all tests.
3. RESULTS
3.1. Patient demographics
The patients’ return rate was 100% in both groups. The mean age of patients was 34.68 ± 9.71 years and 75.0% of them were female whereas 25.0% were male. As shown in Table 1, the patients demographics in terms of gender (P = 0.72, chi square test), the mean age of patients (P = 0.73, two sample t-test), and their VAS pain scores before the treatment (P = 0.37, two sample t test), were not significantly different between the study groups.
3.2. Pain scores
Figure 3 shows the trend of VAS pain scores with respect to time in both groups. The pain decreased immediately after treatment in both groups with a more rapid decrease in Group B at 48 hr. Table 2 gives the means and standard deviations of the scores at the different time in both groups.
| Table 1: Patient demographics | ||||
| Parameter | Group A | Group B | Sig. | |
| Gender | Male | 5 (22.7%) | 6 (27.3%) | 0.72 * |
| Female | 17 (77.3%) | 16 (72.7%) | ||
| Age | 34.68 ± 9.71 | 35.68 ± 10.00 | 0.73** | |
| Preoperative VAS | 6.59 ± 1.76 | 7.05 ± 1.58 | 0.37** | |
| *chi-square test, **two sample t test; Data given as mean ± SD or n (%) | ||||
| Table 2: Mean changes in VAS pain scores over time with treatment. | |||
| Time | Group A | Group B | P-value |
| At 24 hr | 2.82 ± 2.260 | 2.09 ± 2.245 | < 0.001 |
| At 48 hr | 1.55 ± 1.819 | 0.68 ± 1.171 | |
| At 72 hr | 0.55 ± 0.912 | 0.32 ± 0.839 | |
| Data presented as mean ± SD | |||

| Table 3: Two-way repeated measures ANOVA Tests of Within-Subjects and Between-Subjects Effects. | |||||||
| Source | Sum of Squares | df | Mean Square | F | Sig. | Partial Eta Squared | Observed Power |
| Time | 93.197 | 2 | 46.598 | 32.710 | 0.000 | 0.438 | 1.000 |
| Group | 12.121 | 1 | 12.121 | 2.274 | 0.139 | 0.051 | 0.314 |
| Time * Group | 2.470 | 2 | 1.235 | 0.867 | 0.424 | 0.020 | 0.195 |
Table 3 shows the results from the two-way repeated measures ANOVA. The main effect of time on the average VAS scores was found to be statistically significant, F (2,84) = 32.710, P < 0.001, ƞp2=0.438. However, the main effect of treatment group on the average VAS score across time F (1,42) =2.274, P = 0.139, ƞp2 = 0.051, and the interaction effect of Time x Group F (2,84) = 0.867, P = 0.424, ƞp2 = 0.020 were not statistically significant.
The significant main effect of time was further analyzed using the Bonferroni adjusted pairwise comparison for time intervals. Table 4 shows that all pairwise comparisons on the average VAS scores were statistically significant.
3.3. Rescue medicine
Figure 4 shows the rescue medicine intake in both groups during the initial 24 hours post treatment. In Group A, 11 out of 22 participants needed rescue medicine at 4, 5 or 6 hourly intervals. Among these 11, there were 3 who needed another rescue medicine at 18 hours. In Group B, only 4 out of 22 participants needed a single rescue medicine at 4, 6 or 8 hourly intervals, indicating that Group B may have had better pain control during this initial postoperative period.
A two-sample t-test was conducted to analyze the difference in rescue medicine intake between the two groups. The results indicate a significant difference (t(42) = 2.578, P = 0.014) between Group A (M = 0.64, SD = 0.727) and Group B (M = 0.18, SD = 0.395). Group B, on average, took 0.46 rescue medicine less than Group A. The 95% confidence interval of the difference between the means was (0.099, 0.810).
A two-sample t-test was conducted to analyze the difference in rescue medicine intake between the two groups. The results indicate a significant difference (t(42) = 2.578, P = 0.014) between Group A (M = 0.64, SD = 0.727) and Group B (M = 0.18, SD = 0.395). Group B, on average, took 0.46 rescue medicine less than Group A. The 95% confidence interval of the difference between the means was (0.099, 0.810).

| Table 4: Pairwise comparisons at different times. | ||||
| (I) Time | (J) Time | Mean Difference (I-J) | Std. Error | Sig. |
| 24 hr | 48h | 1.341* | 0.263 | 0.000 |
| 72h | 2.023* | 0.304 | 0.000 | |
| 48 hr | 24h | -1.341* | 0.263 | 0.000 |
| 72h | 0.682* | 0.182 | 0.002 | |
| 72 hr | 24h | -2.023* | 0.304 | 0.000 |
| 48h | -0.682* | 0.182 | 0.002 | |
4. DISCUSSION
This study is one of the few research projects18 which compared the effects of NaOCl and Propolis solution as endodontic irrigants on post-operative pain.
Our study aimed to compare the effects of 5.25% sodium hypochlorite (NaOCl) and 20% Propolis solution as endodontic irrigants on post-operative pain in patients with SIP. The findings from this study offer valuable insights into the selection of irrigants and their impact on patient comfort and postoperative pain management.
In the present study, only patients with good systemic health ASA-I and ASA-2 were included, so that the impact of systemic factors on root canal treatment’s outcome and healing process could be excluded as much as possible. And so that any unexpected adverse reactions could be avoided.19
Only premolar teeth with SIP accompanied by clinical symptoms of moderate to severe preoperative pain and with or without tenderness to pressure without apical radiolucency were included. These criteria were specifically chosen for standardization and as these clinical presentations were significant predictors of postoperative pain.19
This study was conducted only in the initial phase of endodontic treatment, so that all patients must receive standard endodontic treatment. Furthermore, it also improved the compliance of patients to follow-up.
Pain was assessed up to 72 h postoperatively because the incidence and severity of post-endodontic pain has been shown to be highest in the first 24 h and decreases substantially to minimal levels.20
Our findings underscore a notable decline in postoperative pain over time for both the NaOCl and Propolis treatment cohorts. This underscores the efficacy of both irrigants in alleviating post-treatment discomfort in patients with SIP, marking a significant positive outcome. Mitigating post-operative pain stands as a pivotal objective in endodontic procedures, elevating the overall patient experience. Similarly, several other studies have also reported positive outcomes with Propolis. Our findings validated those of another study,.18 that compared the effects of propolis nanoparticles and NaOCl as a root canal irrigant on postoperative pain and bacterial reduction in mandibular premolars with necrotic pulps.
Notably, Propolis irrigation has exhibited efficacy in eradicating microbial organisms.21,22 Studies have highlighted Propolis's superiority over calcium hydroxide in combating endodontic infections when used as an intracanal medication,14,23 and in reducing polymicrobial infections in necrotic primary molar root canals.24
The consistent reduction in pain scores observed across both groups at 24-, 48-, and 72-hours post-treatment suggests that Propolis presents itself as a viable substitute for NaOCl as an endodontic irrigant. This discovery holds significant implications, offering clinicians an alternative solution for irrigation, particularly beneficial for patients prone to allergies or sensitivities to NaOCl.25 The growing interest in Propolis within endodontics stems from its documented antimicrobial,24 and anti-inflammatory properties. Notably, it inhibits prostaglandin and leukotriene synthesis, resulting in reduced tissue irritation, attributed in part to the presence of caffeine phenethyl ester.15 Studies employing Propolis have demonstrated its superiority, surpassing even a triple antibiotic blend in combatting microbial organisms like E. faecalis.26 Similarly, our findings lend support to the consideration of Propolis as a promising option in this regard. However, it should be noted that a low concentration of Propolis might limit its efficacy against microbial infection.16
Analgesic administration within the initial 24 hours following SIP treatment remains crucial in addressing patient discomfort.27 Yet, controversies persist regarding the optimal approach to managing post-treatment pain.28 In our investigation, the Propolis group required significantly less rescue analgesics than the NaOCl group. This finding suggests that Propolis may contribute to a more comfortable postoperative experience for patients. The reduced need for rescue medication not only improves patient satisfaction but can also have economic implications by reducing the cost of additional pain management measures.
The strengths of the present study are randomization of both intervention and control groups. This randomized controlled trial demonstrated high internal validity due to its rigorous randomization process, standardized measurement tools, and efforts to control confounding variables such as age, gender, and baseline health status. Secondly, patients and assessors were blinded to the root canal irrigant used, minimizing bias, and ensuring the validity of the study results. Thirdly, the compliance of the patient, there were none lost to follow-up as the study was carried out only in the initial phase of multi visit endodontic treatment.
5. LIMITATIONS
Although our study yields promising findings, it is imperative to recognize its limitations. One notable challenge arose from occasionally compromised visibility within the irrigating field during the irrigation process. The Propolis irrigant had a cloudy sandy appearance, which made it difficult for operators to fully visualize the treatment area. Additionally, instances occurred where the working length required adjustment during the second visit of root canal treatment due to blockages caused by certain components of the Propolis irrigant. Exploring alternative formulations and concentrations of Propolis in the form of clear solutions may alleviate visibility issues during irrigation, thereby potentially enhancing treatment outcomes.
6. CONCLUSION
In conclusion, our study demonstrates that both 5.25% NaOCl and 20% Propolis solution are effective in reducing post-operative pain in patients with SIP. The choice between these two irrigants should consider factors such as patient sensitivities, allergies, and the need for rescue analgesics, where Propolis shows an advantage. These results contribute to the broader discussion on improving patient comfort in endodontic treatments and suggest that Propolis is a promising alternative to traditional NaOCl irrigation. Further research and clinical trials are warranted to explore the applicability of propolis as irrigant in different clinical scenarios.
7. Acknowledgements
The authors would like to thank the pharmaceutical lab for their invaluable input and support throughout the making of Propolis irrigant and the interns posted at the department for their assistance in data collection.
8. Data availability
The numerical data generated in this study is available with the corresponding author and can be requested.
9. Source of funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
10. Conflict of interest statement
The authors have no conflicts of interest to declare.
11. Authors’ contribution
TT: Contributed to conception, design, data acquisition and interpretation, drafted and critically revised the manuscript.
AZR, ZAR: Performed all statistical analyses, drafted, and critically revised the manuscript.
FN: Contributed to concept, design, and critically revised the manuscript.
AH, MM: Contributed to data acquisition and drafted the manuscript.
All authors gave their final approval and agreed to be accountable for all aspects of the work.
12. REFERENCES
- Tampi MP, Pilcher L, Urquhart O, Kennedy E, O'Brien KK, Lockhart PB, et al. Antibiotics for the urgent management of symptomatic irreversible pulpitis, symptomatic apical periodontitis, and localized acute apical abscess: Systematic review and meta-analysis—a report of the American Dental Association. J Am Dent Assoc. 2019 Dec;150(12):e179-e216. DOI: 10.1016/J.ADAJ.2019.09.011
- da Silva EJNL, Monteiro MR, Belladonna FG, Almeida JF, De-Deus G, Neves A de A. Postoperative pain after foraminal instrumentation with a reciprocating system and different irrigating solutions. Braz Dent J. 2015;26(3):216-221. PMID: 31761029 PMCID:PMC8098651 DOI: 1016/j.adaj.2019.09.011
- Raoof M, Sadr A, Nazari F, Amanpour S, Nazeri M, Sadr S, et al. Effect of naproxen on postoperative pain in teeth with irreversible pulpitis. Journal of Basic Research in Medical Sciences. 2015;2(2):37-43. Accessed November 13, 2022. http://jbrms.medilam.ac.ir/article-1-142-en.html
- Cramer JD, Barnett ML, Anne S, et al. Nonopioid, Multimodal Analgesia as First-line Therapy After Otolaryngology Operations: Primer on Nonsteroidal Anti-inflammatory Drugs (NSAIDs). Otolaryngol Head Neck Surg. 2021 Apr;164(4):712-719.DOI: 1177/0194599820947013
- Fricke JR, Hewitt DJ, Jordan DM, Fisher A, Rosenthal NR. A double-blind placebo-controlled comparison of tramadol/acetaminophen and tramadol in patients with postoperative dental pain. Pain. 2004;109(3):250-257. DOI: 1016/j.pain.2004.01.004
- Topçuoğlu HS, Topçuoğlu G, Arslan H. The Effect of Different Irrigation Agitation Techniques on Postoperative Pain in Mandibular Molar Teeth with Symptomatic Irreversible Pulpitis: A Randomized Clinical Trial. J Endod. 2018;44(10):1451-1456. DOI: 1016/j.joen.2018.06.008
- Boutsioukis C, Lambrianidis T, Verhaagen B, et al. The effect of needle-insertion depth on the irrigant flow in the root canal: evaluation using an unsteady computational fluid dynamics model. J Endod. 2010;36(10):1664-1668. DOI: 1016/j.joen.2010.06.023
- Katiyar D. Propolis: A natural biomaterial. Mater Today Proc. Published online June 2, 2023. https://doi.org/10.1016/j.matpr.2023.05.522
- Natarajan K, Singh S, Burke TR, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci U S A. 1996;93(17):9090-9095. DOI: PMID: 8799159 PMCID: PMC38600 DOI: 1073/pnas.93.17.9090
- Tavares JAO, da Silva FA, Santos TML, Caneppele TMF, Augusto MG. The effectiveness of propolis extract in reducing dentin hypersensitivity: A systematic review. Arch Oral Biol. 2021;131:105248. PMID: 34534811 DOI: 1016/j.archoralbio.2021.105248
- Lisbona-González MJ, Muñoz-Soto E, Reyes-Botella C, Olmedo-Gaya MV, Diaz-Castro J, Moreno-Fernandez J. Study of the Antimicrobial Effect of an Ethanolic Extract of Propolis in Periodontal Disease. Applied Sciences 2021, Vol 11, Page 7463. 2021;11(16):7463. DOI: 3390/app11167463
- Hashad Nada, Labib Ahmed, Shaheen N, Ezzat Marwa. Microbial Evaluation Following Two Irrigation-Medication Protocols in Secondary Infection Cases. Egypt Dent J. 2022;68(3):2789-2796. DOI: 21608/edj.2022.127174.2021
- Ravishankar P, Lakshmi T, Pharmaceutical AKJ of, 2011 undefined. Ethno-botanical approach for root canal treatment-an update. Citeseer. Accessed December 18, 2022. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=fc20e880acdd90aadefe83bac6146496872ea365
- Kayaoglu G, Ömürlü H, Akca G, et al. Antibacterial activity of Propolis versus conventional endodontic disinfectants against Enterococcus faecalis in infected dentinal tubules. J Endod. 2011;37(3):376-381. DOI: 1016/J.JOEN.2010.11.024
- Neiva KG, Catalfamo DL, Holliday S, Wallet SM, Pileggi R. Propolis decreases lipopolysaccharide-induced inflammatory mediators in pulp cells and osteoclasts. Dent Traumatol. 2014;30(5):362-367. DOI: 10.1111/EDT.12096
- Poonam Shingare, Vishwas Chaugule. Comparative evaluation of antimicrobial activity of miswak, propolis, sodium hypochlorite and saline as root canal irrigants by microbial culturing and quantification in chronically exposed primary teeth. Germs. 2011;1(1):12-21.
- Bashetty K, Hegde J. Comparison of 2% chlorhexidine and 5.25% sodium hypochlorite irrigating solutions on postoperative pain: a randomized clinical trial. Indian J Dent Res. 2010;21(4):523-527. DOI: 10.4103/0970-9290.74225
- Elaguizy RM, Emara RS, Aly Fahmy R, Mohamed R, Boghdadi E. Effect of Propolis nanoparticles versus sodium hypochlorite as root canal irrigant on postoperative pain and bacterial reduction in mandibular premolars with necrotic pulps: a randomized clinical trial. Egypt Dent J. 2024;70(2):2117-2127. DOI: 10.21608/EDJ.2024.268148.2927
- Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic–explanatory continuum indicator summary (PRECIS): a tool to help trial J Clin Epidemiol. 2009;62(5):464-475. DOI: 10.1016/J.JCLINEPI.2008.12.011
- Shamszadeh S, Shirvani A, Asgary S. Does occlusal reduction reduce post-endodontic pain? A systematic review and meta-analysis. J Oral Rehabil. 2020;47(4):528-535. DOI: 1111/JOOR.12929
- Valera MC, Da Rosa JA, Maekawa LE, et al. Action of propolis and medications against Escherichia coli and endotoxin in root canals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(4). DOI: 1016/J.TRIPLEO.2010.01.029
- Carbajal Mejía JB. Antimicrobial effects of calcium hydroxide, chlorhexidine, and propolis on Enterococcus faecalis and Candida albicans. J Investig Clin Dent. 2014;5(3):194-200. DOI: 1111/jicd.12041
- Shabbir J, Qazi F, Farooqui W, Ahmed S, Zehra T, Khurshid Z. Effect of Chinese Propolis as an Intracanal Medicament on Post-Operative Endodontic Pain: A Double-Blind Randomized Controlled Trial. International Journal of Environmental Research and Public Health 2020, Vol 17, Page 445. 2020;17(2):445. DOI: 3390/IJERPH17020445
- de Rezende GP da SR, da Costa LR de RS, Pimenta FC, Baroni DA. In vitro antimicrobial activity of endodontic pastes with propolis extracts and calcium hydroxide: a preliminary study. Braz Dent J. 2008;19(4):301-305. DOI: 10.1590/S0103-64402008000400003
- Slaughter RJ, Watts M, Vale JA, Grieve JR, Schep LJ. The clinical toxicology of sodium hypochlorite. Clin Toxicol. 2019;57(5):303-311. DOI: 1080/15563650.2018.1543889
- Madhubala MM, Srinivasan N, Ahamed S. Comparative evaluation of propolis and triantibiotic mixture as an intracanal medicament against Enterococcus faecalis. J Endod. 2011;37(9):1287-1289. DOI: 1016/J.JOEN.2011.05.028
- Parirokh M, Sadr S, Nakhaee N, Abbott P V., Manochehrifar H. Comparison between Prescription of Regular or On-demand Ibuprofen on Postoperative Pain after Single-visit Root Canal Treatment of Teeth with Irreversible Pulpitis. J Endod. 2014;40(2):151-154. DOI: 1016/J.JOEN.2013.09.024
- Alhilou AM, Al-Moraissi EA, Bakhsh A, Christidis N, Näsman P. Pain after emergency treatments of symptomatic irreversible pulpitis and symptomatic apical periodontitis in the permanent dentition: a systematic review of randomized clinical trials. Frontiers in Oral Health. 2023;4:1147884. DOI: 10.3389/FROH.2023.1147884/FULL