Neeru Sahni, MD1, Sheetal Masatkar, MD2, Girdhar S Bora, Mch3, Ravimohan S Mavuduru, Mch4
1Assistant Professor; 2Senior Resident,
Department of Anesthesia & Intensive Care, PGIMER, Chandigarh (India)
3Assistant Professor; 4Associate Professor
Department of Urology, PGIMER, Chandigarh (India)
Correspondence: Dr. Neeru Sahni, MD, Assistant Professor, Dept of Anesthesia and Intensive Care, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh 160012 (India); Phone: 09872646106; E-mail : neerunalin@yahoo.com
ABSTRACT
Von Hippel-Lindau (VHL) disease is a disorder with multiple systemic manifestations. We report a case of VHL undergoing robot assisted nephron sparing surgery for recurrent renal cell carcinoma. The anesthetic concerns in perioperative management of a patient with VHL are also described.
Key words: Von Hippel-Lindau disease; Robot-assisted surgery; Carcinoma, Renal Cell
Citation: Sahni N, Masatkar S, Bora GS, Mavuduru RS. Anesthetic management of patient with Von Hippel-Lindau disease undergoing Robot-assisted nephron sparing surgery. Anaesth Pain & Intensive Care 2016;20(1):68-70.
INTRODUCTION
Von Hippel-Lindau (VHL) disease is an autosomal dominant disease which has variable penetrance. The clinical manifestations can vary from cerebellar, medulla oblongata, spinal or retinal hemangioblastoma, pheochromocytoma and renal cell carcinoma.1 We present anesthetic management of a case of VHL disease with cerebellar hemangioblastoma and renal cell carcinoma presenting for robot assisted nephron sparing surgery.
CASE REPORT
Twenty-five year old male patient, weighing 70 kg, a known case of VHL disease was scheduled for robot assisted nephron sparing surgery in view of recurrence of renal cell carcinoma. Patient presented with headache and vomiting 14 years ago and was investigated and diagnosed to have cerebellar hemangioblastoma. He underwent excision of lesion along with ventriculoperitoneal (VP) shunt. Four years later, he presented with abdominal pain and was diagnosed to have right renal cell carcinoma for which right sided nephron sparing surgery was performed. Presently, he had recurrence of renal cell carcinoma and was scheduled for robot assisted repeat nephron sparing surgery on right side.
During pre-anesthetic check-up, he had no current complaints of headache, nausea, vomiting or flushing, suggestive of raised intra-cranial pressure or pheochromocytoma. There was no history of any other comorbid illness. As the last surgery was four years ago, he was again screened for pheochromocytoma which has a common association with VHL. His plasma free metanephrine (71.7 pg/ml), normetanephrine (135.6 pg/ml) and serum cortisol (18.9 µg/ml) levels were normal. Hemogram, thyroid function tests, serum electrolytes and renal function tests were also within normal limits. Contrast enhanced MRI brain revealed no recurrent/residual tumor, well decompressed ventricular function and a shunt tube in situ. Computed tomography of abdomen revealed bilateral renal masses which were hypervascular (Figure 1).
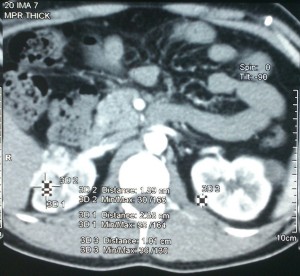
Figure 1: Computed tomography of abdomen revealed bilateral renal masses
Patient was kept nil by mouth for eight hours before surgery. Two large bore intravenous cannulae were placed as the patient was expected to be out of the anesthetist’s approach intraoperatively during robot-assisted surgery. Anesthesia induction was performed with injection morphine 7.5 mg and injection propofol 140 mg IV. Tracheal intubation was facilitated with injection vecuronium 7 mg and anesthesia was maintained with desflurane in a mixture of oxygen and air. Nitrous oxide was avoided because of carbon dioxide pneumoperitoneum. Invasive arterial line was inserted for beat-to-beat monitoring of blood pressure and, for frequent sampling arterial blood for blood gas analysis in a patient undergoing prolonged pneumoperitoneum in a re-do major surgery. Patient was operated in left lateral position. Duration of surgery was 4 hours and that of pneumoperitoneum was 3 hours with about 300 ml blood loss. Intraoperatively, hemodynamic parameters remained stable. At the end of surgery, incision site was infiltrated with 20 ml of 0.2% ropivacaine and injection ondansetron 8 mg was given IV as prophylaxis for postoperative nausea and vomiting. Trachea was extubated after giving injection neostigmine 3.5 mg and glycopyrrolate 0.7 mg. Injection paracetamol 1 gm was given for postoperative pain, every 8 hours and injection fentanyl 50 µg was used as a rescue analgesic. Patient was observed for 48 hours postoperatively and discharged on seventh postoperative day after uneventful postoperative course.
DISCUSSION
VHL is a rare disease with prevalence ranging from 1:35,000 to 1:65,000.2 Various case reports mention cesarean section for patients with VHL.2,3,4,5 The authors have chosen subarachnoid block, epidural anesthesia or general anesthesia depending on associated problems with VHL. However, to the best of our knowledge, this is the first case of VHL undergoing robot-assisted nephron sparing surgery for recurrent renal cell carcinoma. Anesthetic management is based on the associated findings like hemangioblastomas (retinal, spinal, central nervous system), renal cell carcinoma and especially pheochromocytoma as perioperative mortality can be upto 50% if this remains undetected.6 Our patient had already undergone surgery for cerebellar hemangioblastoma with VP shunt and raised ICP or shunt malfunction was ruled out preoperatively. Hallsworth et al have advocated intracranial pressure monitoring in patient with VHL disease and symptomatic cerebellar hemangioblastomas.3 Pheochromocytoma was also ruled out preoperatively clinically as well as with levels of serum metanephrine and nor-metanephrine.
Robot-assisted partial nephrectomy has certain advantages over laparoscopic procedure in terms of reduced blood loss, reduced surgical complications, lesser pain scores and shorter hospital stay. Due to reduction in warm ischemia time, the extent of renal damage is minimized.6,7 Anesthetic considerations in a robot-assisted surgery range from management of physiologic consequences and complications due to prolonged pneumoperitoneum (venous gas embolism, subcutaneous emphysema) to spatial restrictions and patient positioning. Airway pressure concerns are lesser in patient getting operated in lateral position as compared to reverse Trendelenburg position for radical prostatectomy or cystectomy. Patient is positioned away from the anesthetist’s approach and hence, the need for adequate intravenous access with long tubings for extension.8 Also, in view of the expected long duration of pneumoperitoneum in re-do surgery where adhesions are expected, we felt the need for inserting arterial line so as to get intraoperative arterial blood gas analysis. Swelling of face and conjunctival chemosis on the dependent side of face is not uncommon. The issue of cerebral edema and raised intra-ocular pressure as well as airway edema is less due to lateral position in partial nephrectomy. As any intraoperative emergency cannot be managed without undocking the robotic arms which takes time, it is suggested that drills should be done to train the surgeons in quick undocking. Perioperative management of patients undergoing robot-assisted procedure needs good team communication between anesthetist and surgeon.9
Thus, a patient with VHL needs thorough work up to diagnose the associated lesions in various organ systems as the presence of these associated lesions is a major factor determining the choice of anesthesia. Robot-assisted nephron sparing surgery is beneficial provided the team is trained to manage any intraoperative mishap.
Conflict of interest: None declared by the authors
REFERENCES
1Assistant Professor; 2Senior Resident,
Department of Anesthesia & Intensive Care, PGIMER, Chandigarh (India)
3Assistant Professor; 4Associate Professor
Department of Urology, PGIMER, Chandigarh (India)
Correspondence: Dr. Neeru Sahni, MD, Assistant Professor, Dept of Anesthesia and Intensive Care, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh 160012 (India); Phone: 09872646106; E-mail : neerunalin@yahoo.com
ABSTRACT
Von Hippel-Lindau (VHL) disease is a disorder with multiple systemic manifestations. We report a case of VHL undergoing robot assisted nephron sparing surgery for recurrent renal cell carcinoma. The anesthetic concerns in perioperative management of a patient with VHL are also described.
Key words: Von Hippel-Lindau disease; Robot-assisted surgery; Carcinoma, Renal Cell
Citation: Sahni N, Masatkar S, Bora GS, Mavuduru RS. Anesthetic management of patient with Von Hippel-Lindau disease undergoing Robot-assisted nephron sparing surgery. Anaesth Pain & Intensive Care 2016;20(1):68-70.
INTRODUCTION
Von Hippel-Lindau (VHL) disease is an autosomal dominant disease which has variable penetrance. The clinical manifestations can vary from cerebellar, medulla oblongata, spinal or retinal hemangioblastoma, pheochromocytoma and renal cell carcinoma.1 We present anesthetic management of a case of VHL disease with cerebellar hemangioblastoma and renal cell carcinoma presenting for robot assisted nephron sparing surgery.
CASE REPORT
Twenty-five year old male patient, weighing 70 kg, a known case of VHL disease was scheduled for robot assisted nephron sparing surgery in view of recurrence of renal cell carcinoma. Patient presented with headache and vomiting 14 years ago and was investigated and diagnosed to have cerebellar hemangioblastoma. He underwent excision of lesion along with ventriculoperitoneal (VP) shunt. Four years later, he presented with abdominal pain and was diagnosed to have right renal cell carcinoma for which right sided nephron sparing surgery was performed. Presently, he had recurrence of renal cell carcinoma and was scheduled for robot assisted repeat nephron sparing surgery on right side.
During pre-anesthetic check-up, he had no current complaints of headache, nausea, vomiting or flushing, suggestive of raised intra-cranial pressure or pheochromocytoma. There was no history of any other comorbid illness. As the last surgery was four years ago, he was again screened for pheochromocytoma which has a common association with VHL. His plasma free metanephrine (71.7 pg/ml), normetanephrine (135.6 pg/ml) and serum cortisol (18.9 µg/ml) levels were normal. Hemogram, thyroid function tests, serum electrolytes and renal function tests were also within normal limits. Contrast enhanced MRI brain revealed no recurrent/residual tumor, well decompressed ventricular function and a shunt tube in situ. Computed tomography of abdomen revealed bilateral renal masses which were hypervascular (Figure 1).

Figure 1: Computed tomography of abdomen revealed bilateral renal masses
Patient was kept nil by mouth for eight hours before surgery. Two large bore intravenous cannulae were placed as the patient was expected to be out of the anesthetist’s approach intraoperatively during robot-assisted surgery. Anesthesia induction was performed with injection morphine 7.5 mg and injection propofol 140 mg IV. Tracheal intubation was facilitated with injection vecuronium 7 mg and anesthesia was maintained with desflurane in a mixture of oxygen and air. Nitrous oxide was avoided because of carbon dioxide pneumoperitoneum. Invasive arterial line was inserted for beat-to-beat monitoring of blood pressure and, for frequent sampling arterial blood for blood gas analysis in a patient undergoing prolonged pneumoperitoneum in a re-do major surgery. Patient was operated in left lateral position. Duration of surgery was 4 hours and that of pneumoperitoneum was 3 hours with about 300 ml blood loss. Intraoperatively, hemodynamic parameters remained stable. At the end of surgery, incision site was infiltrated with 20 ml of 0.2% ropivacaine and injection ondansetron 8 mg was given IV as prophylaxis for postoperative nausea and vomiting. Trachea was extubated after giving injection neostigmine 3.5 mg and glycopyrrolate 0.7 mg. Injection paracetamol 1 gm was given for postoperative pain, every 8 hours and injection fentanyl 50 µg was used as a rescue analgesic. Patient was observed for 48 hours postoperatively and discharged on seventh postoperative day after uneventful postoperative course.
DISCUSSION
VHL is a rare disease with prevalence ranging from 1:35,000 to 1:65,000.2 Various case reports mention cesarean section for patients with VHL.2,3,4,5 The authors have chosen subarachnoid block, epidural anesthesia or general anesthesia depending on associated problems with VHL. However, to the best of our knowledge, this is the first case of VHL undergoing robot-assisted nephron sparing surgery for recurrent renal cell carcinoma. Anesthetic management is based on the associated findings like hemangioblastomas (retinal, spinal, central nervous system), renal cell carcinoma and especially pheochromocytoma as perioperative mortality can be upto 50% if this remains undetected.6 Our patient had already undergone surgery for cerebellar hemangioblastoma with VP shunt and raised ICP or shunt malfunction was ruled out preoperatively. Hallsworth et al have advocated intracranial pressure monitoring in patient with VHL disease and symptomatic cerebellar hemangioblastomas.3 Pheochromocytoma was also ruled out preoperatively clinically as well as with levels of serum metanephrine and nor-metanephrine.
Robot-assisted partial nephrectomy has certain advantages over laparoscopic procedure in terms of reduced blood loss, reduced surgical complications, lesser pain scores and shorter hospital stay. Due to reduction in warm ischemia time, the extent of renal damage is minimized.6,7 Anesthetic considerations in a robot-assisted surgery range from management of physiologic consequences and complications due to prolonged pneumoperitoneum (venous gas embolism, subcutaneous emphysema) to spatial restrictions and patient positioning. Airway pressure concerns are lesser in patient getting operated in lateral position as compared to reverse Trendelenburg position for radical prostatectomy or cystectomy. Patient is positioned away from the anesthetist’s approach and hence, the need for adequate intravenous access with long tubings for extension.8 Also, in view of the expected long duration of pneumoperitoneum in re-do surgery where adhesions are expected, we felt the need for inserting arterial line so as to get intraoperative arterial blood gas analysis. Swelling of face and conjunctival chemosis on the dependent side of face is not uncommon. The issue of cerebral edema and raised intra-ocular pressure as well as airway edema is less due to lateral position in partial nephrectomy. As any intraoperative emergency cannot be managed without undocking the robotic arms which takes time, it is suggested that drills should be done to train the surgeons in quick undocking. Perioperative management of patients undergoing robot-assisted procedure needs good team communication between anesthetist and surgeon.9
Thus, a patient with VHL needs thorough work up to diagnose the associated lesions in various organ systems as the presence of these associated lesions is a major factor determining the choice of anesthesia. Robot-assisted nephron sparing surgery is beneficial provided the team is trained to manage any intraoperative mishap.
Conflict of interest: None declared by the authors
REFERENCES
- Lindau A. Studies on cysts in cerebellum: structure, pathogenesis and relations to angiomatosis of retina. Acta Path Microbiol Scand. 1926;1:128.
- Wang A, Sinatra RS. Epidural anesthesia for cesarean section in a patient with von Hippel-Lindau disease and multiple sclerosis. Anesth Analg. 1999;88:1083-4. [PubMed]
- Hallsworth D, Thompson J, Wilkinson D, Kerr S, Russell R. Intracranial pressure monitoring and caesarean section in a patient with von Hippel-Lindau disease and symptomatic cerebellar haemangioblastoma. Int J Obstet Anesth. 2015;24:73-7.
- Boker A, Ong BY. Anesthesia for cesarean section and posterior fossa craniotomy in a patient with von Hippel-Lindau disease. Can J Anaesth. 2001;48:387-90. [PubMed]
- Matthews AJ, Halshaw J. Epidural anaesthesia in von Hippel-Lindau disease. Management of childbirth and anaesthesia for caesarean section. Anaesthesia. 1986;41:853-55. [PubMed]
- Png KS, Sundaram CP. Current status of robot-assisted laparoscopic partial nephrectomy. Indian J Surg Oncol. 2012;3:91-5. doi: 10.1007/s13193-011-0092-4. [PubMed] [Free full text]
- Minervini A, Vittori G, Antonelli A, Celia A, Crivellaro S, Dente D, et al. Open versus robotic-assisted partial nephrectomy: a multicenter comparison study of perioperative results and complications. World J Urol 2014;32:287-93. [PubMed]
- Lee JR. Anesthetic considerations for robotic surgery. Korean J Anesthesiol. 2014;66:3-11. doi: 10.4097/kjae.2014.66.1.3 [PubMed] [Free full text]
- Kaye AD, Vadivelu N, Ahuja N, Mitra S, Silasi D, Urman RD. Anesthetic considerations in robotic-assisted gynecologic surgery. Ochsner J. 2013;13:517-24. [PubMed] [Free full text]