Rozheen Shukri Karam1, Fouad K. Mohammad2*
Author affiliation:
Background & Objective: There is an increased ratio of cesarean sections (CS) compared to normal deliveries the world over. Both general and regional anesthetic techniques are in practice for CS. Limited information is available on the determination of blood cholinesterase (ChE) activity in conjunction with oxidative stress biomarkers after the CS. The purpose of the present study was to determine and compare the plasma and erythrocyte ChE activity as well as malondialdehyde (MDA) level and total antioxidant status in pregnant women before anesthesia and after the use of general anesthesia (GA) or spinal anesthesia (SA) for elective CS.
Methodology: A pre-post study was conducted on 102 full-term pregnant women, who underwent elective CS in four hospitals at Duhok (Iraq) from January 2022 to July 2022. GA with propofol and SA with bupivacaine 0.5% were administered to 52 and 50 women, respectively. Maternal blood samples before anesthesia and after the completion of operation were assayed for the ChE activity in the plasma and erythrocytes, and plasma oxidative stress biomarkers malondialdehyde and total antioxidant status.
Results: Plasma ChE activity significantly decreased in the GA and SA groups after the cesarean-deliveries compared to the corresponding pre-anesthetic values. The plasma MDA level in both GA and SA groups, but not the total antioxidant status, was significantly reduced. Estimation of the odds ratio and the relative risk indicated that a risk factor is associated with the reduced plasma ChE activity in women after the CS when the general anesthetic propofol is used.
Conclusion: Both general anesthesia with propofol and spinal anesthesia with bupivacaine inhibited plasma cholinesterase activity and reduced oxidative stress biomarker malondialdehyde after the cesarean sections.
Abbreviations: CS - Cesarean section; ChE: Cholinesterase; EChE: Erythrocyte Cholinesterase; MDA: Malondialdehyde; OS: Oxidative Stress; PChE: Plasma ChE; TAS: Total Antioxidant Status.
Key words: Anesthesia, Spinal; Cesarean section; General anesthesia; Malondialdehyde; Oxidative stress
Citation: Karam RS, Mohammad FK. Changes in blood oxidative stress biomarker and cholinesterase activity after general vs spinal anesthesia for elective cesarean sections. Anaesth. pain intensive care 2023;27(3):396−404; DOI: 10.35975/apic.v27i3.2244
Received: March 23, 2023; Reviewed: May 06, 2023; Accepted: May 09, 2023
Elective cesarean section (CS) or cesarean delivery has gained high preference compared to the vaginal birth among women in many countries.1−4 For this purpose, either general anesthetics are used with propofol or other injectable anesthetics, or the spinal or epidural anesthesia with local anesthetics such as bupivacaine and lidocaine.3−6
Many studies have attempted to examine the pharmaco-biochemical outcome, including blood cholinesterase (ChE) activity and oxidative stress (OS) biomarkers after different anesthetics.7−12 Any change in these variables could be attributed to the surgical procedures applied and their durations, as well as to the type(s) of anesthesia and anesthetics-drug combinations used.8,10,13,14 Most importantly, several reports showed that blood cholinesterase (ChE) activities of the plasma (PChE) and erythrocytes (EChE) and OS indices such as plasma malondialdehyde (MDA) level and total antioxidant status (TAS) or total antioxidant capacity (TAC) as well as other biochemical variables, can be affected by normal physiological changes that occur during pregnancy, especially in the period close to delivery.15−17
What complicates the matter is the fact that during CS, there is enhancement of OS markers which may be due to physical and emotional stress, as well as due to anesthetic drugs used.18,19 On the other hand, in vitro and in vivo evidence suggests that the commonly used anesthetic propofol possesses antioxidant properties,20−22 with cholinergic implications in its anesthetic action.22,23 Local anesthetics such as lidocaine and bupivacaine were also reported to possess anti-ChE properties, especially on PChE activity.24 However, limited information is available on blood ChE activity in conjunction with OS changes after the CS in the light of the expected profuse blood loss and changes in blood biochemical variables.25−27 The purpose of the present study was to determine PChE and EChE activities as well as plasma MDA level and TAS in pregnant women before anesthesia and after the use of GA or SA for elective CS.
Ethical approval was obtained from the Committee of Post Graduate Studies, College of Pharmacy, University of Duhok, KRG, Iraq (No. 470, October 6, 2021) and from the Research Ethics Committee, Duhok Directorate General of Health, Duhok, KRG, Iraq (No. 10112021-11-17, November 10, 2021). All women in the study were informed about the study purpose, nature of blood sampling procedures to be used and the expected outcome of the study; a written consent was obtained from each one.
2.1. Inclusion and exclusion criteria
Full term pregnant women were considered for the study when they underwent elective CS under general or spinal anesthesia in four different hospitals in Duhok, KRG, Iraq. Their ages ranged from 20 to 45 y. Emergency cases of CS or those of multiple pregnancy were excluded from the present study.
2.2. Study design and participants
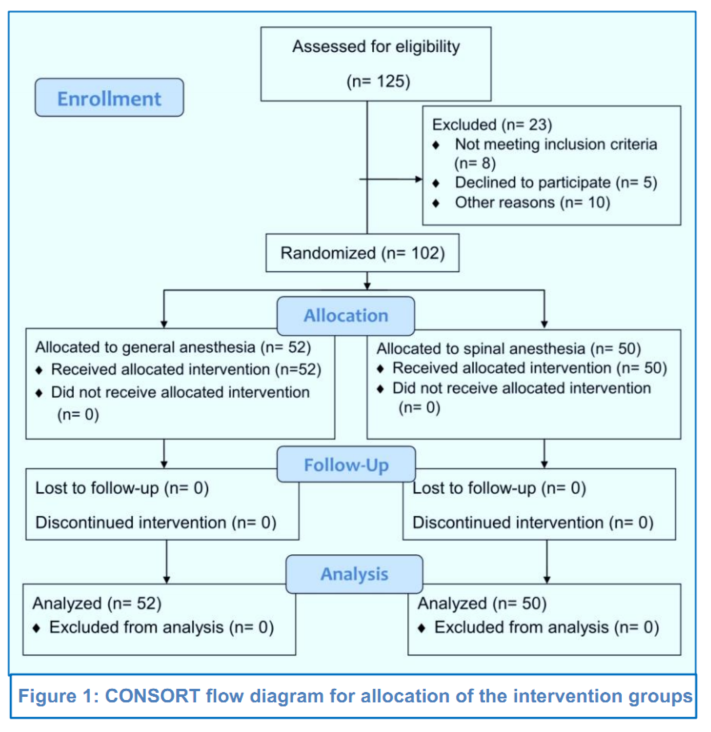
In a pretest-posttest study design (https://explorable.com/pretest-posttest-designs), the study was conducted from January 2022 to July 2022. The included and excluded participants in the study are shown in the CONSORT flow diagram (Figure 1). Accordingly, 102 women were finally allocated into two intervention groups, based on the type of anesthetics used, which were the Group GA to receive GA with propofol (n = 52) or the Group SA to undergo SA with 0.5% bupivacaine (n = 50) for the elective CS.
Sample size estimation for clinical research set by Benchmarksixsigma.com was used, considering a confidence level of 95%, power of the test 80%, and 20% difference of PChE (before anesthesia vs. after operation) which is statistically the most significant measurement with a 5% margin of error (alpha).
2.3. Anesthesia protocols
A specialized surgical and anesthetic team from each hospital managed the GA, SA, and the CS in a similar manner during the course of the study.
2.3.1. General anesthesia
Participants received 1% propofol 2-2.5 mg/kg IV for the induction of GA in the standard dose for weight basis. The propofol brands used were from different manufacturers, including, Propofol-PF 1%, Polifarma, Istanbul, Turkiye, Somnopol, Aversi, Tbilisi, Georgia, and Propofol 1%, Fresenius, Fresenius Kabi, Graz, Austria. Non-depolarizing neuromuscular blockers were used for endotracheal intubation. They were atracurium (Atacure, Hikma Pharmaceuticals, Amman, Jordan) or rocuronium (Rocuronium Kabi, Fresenius Kabi, Graz, Austria). The maintenance of the general anesthesia was done by using isoflurane (Floran, Hikma Pharmaceuticals, Amman, Jordan).
2.3.2. Spinal anesthesia
Participants received intrathecal 0.5% bupivacaine 7.5 mg for spinal anesthesia. Bupivacaine used was from different manufacturing sources, which were: Bupivacaine, Aguettant Corporate, Lyon, France, Buvasin, Vem Ilac, Istanbul, Turkiye, or Marcaine, Aspen, Turkiye
2.4. Blood sampling
Approximately 5 mL of venous blood sample was collected in heparinized tubes from each patient, before anesthesia and immediately after the end of the CS, before the administration of any drug such as neostigmine and atropine in the Group GA. The period between the two blood samples was 30.3 ± 11.3 min for GA and 22.1 ± 8.7 min for SA. The blood samples were centrifuged at 1037 g for 15 min to separate the plasma and erythrocytes which were kept at –20 ºC pending ChE, MDA and TAS assays within 2−3 weeks.
2.4.1. Blood ChE activity
An electrometric method was used to determine PChE and EChE activities using 0.2 mL samples and 0.1 mL of 7.1% acetylcholine iodide as a substrate with a reaction time of 20 min.28,29 The enzymatic activity was expressed as D pH/20 min.
2.4.2. Plasma MDA level
Plasma MDA level was determined by a spectrophotometric method at 535 nm,30 with minor modification as described before.31
2.4.3. Plasma TAS
A spectrophotometric kit (Elabscience Biotechnology Inc., USA) was used to determine plasma TAS using a microplate reader (ELx800, Biotek, USA) at 660 nm.
3. Statistical analysis
All data were statistically analyzed using the statistical software program PAST4.09. Paired Student’s t- test was used for comparison of mean values of pre-anesthesia and post-CS. Correlation was also determined among the variables. Odds ratio and risk ratio were estimated for cases of observed and expected low PChE activity at 20% reductions.31 The level of statistical significance was at P < 0.05.
The demographic data as well as the surgical operation characteristics (duration of operation, and time to recovery from anesthesia) of 102 participants who underwent elective CS are given in Table 1. The mean age in years of both GA and SA groups was (29.1 ± 5.5). The duration of surgery for Group GA was significantly longer than that of the Group SA, and the recovery time from the GA was substantially shorter than that of the SA.
3.1. Blood ChE activity
PChE activities before anesthesia and after the surgery in Group GA and Group SA were measured. In both groups, the PChE activity was significantly (P < 0.05) reduced after the CS in comparison to pre-anesthesia values by 17% and 11%, respectively. However, there was no significant difference in EChE activity in both groups with respect to their pre-anesthesia values (Table 2).
3.2. Plasma oxidative stress biomarkers
In both groups the plasma MDA level was significantly (P < 0.05) reduced after the operation in comparison to the pre-anesthesia values. MDA level reduced by 13% and 24%, in Group GA and Group SA, respectively. However, there was no significant difference in the TAS
in both groups with respect to their pre-anesthesia values (Table 3).
3.3. Risks of reduced PChE after the CS
We constructed a table of odds ratio (Table 4) using the frequency of distribution of PChE activity values (Δ pH/20 min) ≤ 0.62 for the GA and ≤ 0.63 for SA in women who underwent CS. It was noticed that estimation of the odds ratio and the risk ratio indicated a risk factor of reduced PChE activity in women who underwent CS with the use the general anesthetic propofol (Table 5).
3.4. Correlation between PChE, EChE and MDA
There was no correlation between PChE vs. EChE (r = -0.086), PChE vs. MDA (r = 0.130) and EChE vs. MDA (r = -0.23) in women set undergoing elective CS and either GA or SA, taking into account the pre-anesthetic and after the operation ChE activities.
Current evidence strongly suggests an increasing preference of parturients for elective cesarean-delivery, apparently to avoid labor pains and the stress related to normal delivery, in many regions around the world,1−3,32,33 including Iraq.4,34 For elective CS, the most preferred GA is with propofol and SA with bupivacaine. The present study was conducted in four hospitals in Duhok, Iraq, to examine the effects of these two most commonly used anesthetics, on blood OS biomarker and ChE activity. The most prominent finding of the present study was a reduction in PChE activity post-operatively in both types of anesthesia. This enzyme is found in the liver, plasma and other tissues, and it is involved in the metabolism of some anesthetics and related muscle relaxants.35,36 Reduced PChE activity is a biomarker of exposure to pesticides,29,31,37 and it has been implicated in different cardiovascular and hemodynamic imbalances during anesthesia, especially at the level of the autonomic nervous system.9,24,38
The mean PChE activities (Δ pH/20min) before anesthesia were 0.75 and 0.71 in GS and SA, respectively. These values are generally below normal ranges reported by others,28,29 using the same electrometric method we applied in the present study. This could be related to physiological hemodynamic changes expected during pregnancy.35,39-42 Within this context, the results of the present study demonstrated that PChE is further reduced in both GA and SA groups after the CS, and therefore, caution should be practiced during application of anesthetics and neuromuscular blocking agents in surgical operations for the CS. Additionally, it is reasonable to suggest that monitoring blood ChE activity should be a routine integration of maternal health assessment before any CS.
The reduction in PChE activity after the CS in the GA group could be due to the direct effect of propofol. Propofol has been reported to interact with central cholinergic system, during GA, possibly by reducing acetylcholine release and cholinergic functions that are related to anesthesia-induced immobility, amnesia and loss of consciousness.43 In support of this notion,
physostigmine reverses propofol-induced unconsciousness in human volunteers,44 and its pretreatment increases the dose of propofol needed to induce unconsciousness in patients.45 On the other hand, the local anesthetic bupivacaine was reported to possess an inhibitory action over PChE activity.46 This effect could explain the reduction in PChE activity in the SA group of the present study.
Alterations in serum ChE activity were not attributed to the bupivacaine spinal application alone in patients undergoing lower limb surgery, and they were rather attributed in part to surgery-related depression in cellular secretory function as well as to protein depletion due to blood loss.24 It should be stressed here, that we cannot rule out the possible effects of adjuvants, maintenance anesthetics and other medications on PChE activity when used intra-operatively during the CS. Such a reduction of PChE activity after the anesthesia calls for careful hemodynamic support during CS.7,9,24,35,39,42
As reported earlier, EChE activity increases during pregnancy, and this was also true in the present pre-anesthetic activity of this enzyme when compared to normal values reported in non-pregnant women using the same electrometric method.28,29 However, in contrast to PChE, there was no significant difference between the pre-anesthesia and post-CS values of EChE activity in the present study, suggesting a differential effect of both propofol and bupivacaine on PChE vs. EChE. This finding is also in accordance with a previous report about the selective inhibition of butyryl ChE (vs. true ChE) by bupivacaine.47 However, in the study of Gramavy,22 there was a significant reduction of EChE activity with propofol in non-pregnant women as well as in males. This discrepancy could be related to the fact that in the present study the women were in the last stage of pregnancy and were very close to the due date of delivery, which is known to be accompanied by various metabolic changes, including activities of PChE and EChE.39,40,48
Pregnant women who undergo CS are at high risk of suffering from OS due to the physical and emotional stress of surgery and the anesthesia.19 In the present study, pre-anesthesia (GA) levels of plasma MDA significantly decreased after the CS, suggesting a reduction in the OS status of the women.
Several factors may have contributed to the present finding; most importantly, propofol, being an antioxidant, reduces the impact of OS, probably through inhibition of nuclear factor kappa B (NF-κB) signal transduction pathway in liver.49 The plasma OS biomarker MDA, is a byproduct of lipid peroxidation of cell membranes, which also occurs during pregnancy.50,51
It has been reported that propofol results in inhibition of lipid peroxidation and enhancement of antioxidant capacity.49,52 The antioxidant effect of propofol could be due to the phenolic structure of the drug which is similar to α-tocopherol, an antioxidant.53 While there was no significant effect at the TAS level with propofol in the present study, the results suggest a specific effect of the anesthetic at the level of MDA mechanism in pregnant women who underwent CS. However, TAS is the result of overall contributions of several antioxidant mechanisms such as albumin, iron, bilirubin, uric acid;54,55 and it is possible that all these variables were not affected considerably enough by the present CS/anesthetic paradigm.
Similar to the effect of propofol (reduction of the plasma MDA level) in the present study, SA with bupivacaine also significantly reduced MDA level, but not that of TAS after the CS. This finding is reported at present as the first of its kind, though its mechanism is not yet clear. However, it is conceivable to deduce that removing the burden of pregnancy by the CS might have contributed to the antioxidant properties of both propofol and bupivacaine. This effect, however, needs further follow up clinically.
There was no correlation between the PChE and EChE activity, as well as between PChE activity and MDA level, although the PChE activity and MDA values were affected by anesthetics in the present study. This was also true about EChE activity and MDA level. This finding correlates with that of Garmavy22 who found also poor correlation (r = 0.323) between PChE and EChE activity in patients who received propofol. In contrast, a study in pregnant women with gestational diabetes reported a negative correlation between serum ChE activity and the MDA level and a strong correlation between this enzyme and total antioxidant activity in the serum;56 apparently serum ChE acted as a scavenger of free radicals in the presence of OS.
Extended follow up and interval blood sampling was needed; however, none of the participants were willing to pursue the study beyond the CS. The choices of anesthetics (general or local) were limited in the hospitals, probably because of their availability and the preferences of the surgeons. Otherwise, it would have been interesting to study other anesthetics that can be applied in the CS.
Propofol and bupivacaine were used for general anesthesia and spinal anesthesia respectively, for elective cesarean sections in Duhok, Iraq. Inhibition of plasma ChE activity and reduced oxidative stress biomarker MDA occurred differentially after the cesarean sections under GA and SA; with the possibility of health risk association with reduced plasma ChE in case of using propofol. Clinical follow-up studies on the variables used are warranted on women after the cesarean sections.
7. Data availability
The numerical data of this study is available with the first author.
8. Conflict of interests
The authors declare no conflicts of interest with respect to the authorship and/or publication of this article.
9. Acknowledgments
This study was supported by the University of Duhok.
10. Authors’ contribution
RSK: Dealt with the participants, obtained blood samples and executed laboratory assays; conducted literature search, statistical analyses and shared in drafting the manuscript.
FKM: Conceptualized and supervised the study, shared in literature search, statistical analyses and drafting the manuscript. Both authors have read the manuscript and approve it for publication.
Author affiliation:
- Rozheen Shukri Karam, Department of Pharmacology, College of Pharmacy, University of Duhok, KRG, Iraq; E-mail: rozheen.karam@gmail.com
- Fouad K. Mohammad, Department of Physiology, Biochemistry and Pharmacology, College of Veterinary Medicine, University of Mosul, Mosul, Iraq; E-mail: fouadmohammad@yahoo.com; ORCID: {0000-0002-5715-4823}
ABSTRACT
Background & Objective: There is an increased ratio of cesarean sections (CS) compared to normal deliveries the world over. Both general and regional anesthetic techniques are in practice for CS. Limited information is available on the determination of blood cholinesterase (ChE) activity in conjunction with oxidative stress biomarkers after the CS. The purpose of the present study was to determine and compare the plasma and erythrocyte ChE activity as well as malondialdehyde (MDA) level and total antioxidant status in pregnant women before anesthesia and after the use of general anesthesia (GA) or spinal anesthesia (SA) for elective CS.
Methodology: A pre-post study was conducted on 102 full-term pregnant women, who underwent elective CS in four hospitals at Duhok (Iraq) from January 2022 to July 2022. GA with propofol and SA with bupivacaine 0.5% were administered to 52 and 50 women, respectively. Maternal blood samples before anesthesia and after the completion of operation were assayed for the ChE activity in the plasma and erythrocytes, and plasma oxidative stress biomarkers malondialdehyde and total antioxidant status.
Results: Plasma ChE activity significantly decreased in the GA and SA groups after the cesarean-deliveries compared to the corresponding pre-anesthetic values. The plasma MDA level in both GA and SA groups, but not the total antioxidant status, was significantly reduced. Estimation of the odds ratio and the relative risk indicated that a risk factor is associated with the reduced plasma ChE activity in women after the CS when the general anesthetic propofol is used.
Conclusion: Both general anesthesia with propofol and spinal anesthesia with bupivacaine inhibited plasma cholinesterase activity and reduced oxidative stress biomarker malondialdehyde after the cesarean sections.
Abbreviations: CS - Cesarean section; ChE: Cholinesterase; EChE: Erythrocyte Cholinesterase; MDA: Malondialdehyde; OS: Oxidative Stress; PChE: Plasma ChE; TAS: Total Antioxidant Status.
Key words: Anesthesia, Spinal; Cesarean section; General anesthesia; Malondialdehyde; Oxidative stress
Citation: Karam RS, Mohammad FK. Changes in blood oxidative stress biomarker and cholinesterase activity after general vs spinal anesthesia for elective cesarean sections. Anaesth. pain intensive care 2023;27(3):396−404; DOI: 10.35975/apic.v27i3.2244
Received: March 23, 2023; Reviewed: May 06, 2023; Accepted: May 09, 2023
1. INTRODUCTION
Elective cesarean section (CS) or cesarean delivery has gained high preference compared to the vaginal birth among women in many countries.1−4 For this purpose, either general anesthetics are used with propofol or other injectable anesthetics, or the spinal or epidural anesthesia with local anesthetics such as bupivacaine and lidocaine.3−6

Many studies have attempted to examine the pharmaco-biochemical outcome, including blood cholinesterase (ChE) activity and oxidative stress (OS) biomarkers after different anesthetics.7−12 Any change in these variables could be attributed to the surgical procedures applied and their durations, as well as to the type(s) of anesthesia and anesthetics-drug combinations used.8,10,13,14 Most importantly, several reports showed that blood cholinesterase (ChE) activities of the plasma (PChE) and erythrocytes (EChE) and OS indices such as plasma malondialdehyde (MDA) level and total antioxidant status (TAS) or total antioxidant capacity (TAC) as well as other biochemical variables, can be affected by normal physiological changes that occur during pregnancy, especially in the period close to delivery.15−17
What complicates the matter is the fact that during CS, there is enhancement of OS markers which may be due to physical and emotional stress, as well as due to anesthetic drugs used.18,19 On the other hand, in vitro and in vivo evidence suggests that the commonly used anesthetic propofol possesses antioxidant properties,20−22 with cholinergic implications in its anesthetic action.22,23 Local anesthetics such as lidocaine and bupivacaine were also reported to possess anti-ChE properties, especially on PChE activity.24 However, limited information is available on blood ChE activity in conjunction with OS changes after the CS in the light of the expected profuse blood loss and changes in blood biochemical variables.25−27 The purpose of the present study was to determine PChE and EChE activities as well as plasma MDA level and TAS in pregnant women before anesthesia and after the use of GA or SA for elective CS.
2. METHODOLOGY
Ethical approval was obtained from the Committee of Post Graduate Studies, College of Pharmacy, University of Duhok, KRG, Iraq (No. 470, October 6, 2021) and from the Research Ethics Committee, Duhok Directorate General of Health, Duhok, KRG, Iraq (No. 10112021-11-17, November 10, 2021). All women in the study were informed about the study purpose, nature of blood sampling procedures to be used and the expected outcome of the study; a written consent was obtained from each one.
2.1. Inclusion and exclusion criteria
Full term pregnant women were considered for the study when they underwent elective CS under general or spinal anesthesia in four different hospitals in Duhok, KRG, Iraq. Their ages ranged from 20 to 45 y. Emergency cases of CS or those of multiple pregnancy were excluded from the present study.
2.2. Study design and participants
In a pretest-posttest study design (https://explorable.com/pretest-posttest-designs), the study was conducted from January 2022 to July 2022. The included and excluded participants in the study are shown in the CONSORT flow diagram (Figure 1). Accordingly, 102 women were finally allocated into two intervention groups, based on the type of anesthetics used, which were the Group GA to receive GA with propofol (n = 52) or the Group SA to undergo SA with 0.5% bupivacaine (n = 50) for the elective CS.
Sample size estimation for clinical research set by Benchmarksixsigma.com was used, considering a confidence level of 95%, power of the test 80%, and 20% difference of PChE (before anesthesia vs. after operation) which is statistically the most significant measurement with a 5% margin of error (alpha).
2.3. Anesthesia protocols
A specialized surgical and anesthetic team from each hospital managed the GA, SA, and the CS in a similar manner during the course of the study.
2.3.1. General anesthesia
Participants received 1% propofol 2-2.5 mg/kg IV for the induction of GA in the standard dose for weight basis. The propofol brands used were from different manufacturers, including, Propofol-PF 1%, Polifarma, Istanbul, Turkiye, Somnopol, Aversi, Tbilisi, Georgia, and Propofol 1%, Fresenius, Fresenius Kabi, Graz, Austria. Non-depolarizing neuromuscular blockers were used for endotracheal intubation. They were atracurium (Atacure, Hikma Pharmaceuticals, Amman, Jordan) or rocuronium (Rocuronium Kabi, Fresenius Kabi, Graz, Austria). The maintenance of the general anesthesia was done by using isoflurane (Floran, Hikma Pharmaceuticals, Amman, Jordan).
2.3.2. Spinal anesthesia
Participants received intrathecal 0.5% bupivacaine 7.5 mg for spinal anesthesia. Bupivacaine used was from different manufacturing sources, which were: Bupivacaine, Aguettant Corporate, Lyon, France, Buvasin, Vem Ilac, Istanbul, Turkiye, or Marcaine, Aspen, Turkiye
2.4. Blood sampling
Approximately 5 mL of venous blood sample was collected in heparinized tubes from each patient, before anesthesia and immediately after the end of the CS, before the administration of any drug such as neostigmine and atropine in the Group GA. The period between the two blood samples was 30.3 ± 11.3 min for GA and 22.1 ± 8.7 min for SA. The blood samples were centrifuged at 1037 g for 15 min to separate the plasma and erythrocytes which were kept at –20 ºC pending ChE, MDA and TAS assays within 2−3 weeks.
2.4.1. Blood ChE activity
An electrometric method was used to determine PChE and EChE activities using 0.2 mL samples and 0.1 mL of 7.1% acetylcholine iodide as a substrate with a reaction time of 20 min.28,29 The enzymatic activity was expressed as D pH/20 min.
2.4.2. Plasma MDA level
Plasma MDA level was determined by a spectrophotometric method at 535 nm,30 with minor modification as described before.31
2.4.3. Plasma TAS
A spectrophotometric kit (Elabscience Biotechnology Inc., USA) was used to determine plasma TAS using a microplate reader (ELx800, Biotek, USA) at 660 nm.
3. Statistical analysis
All data were statistically analyzed using the statistical software program PAST4.09. Paired Student’s t- test was used for comparison of mean values of pre-anesthesia and post-CS. Correlation was also determined among the variables. Odds ratio and risk ratio were estimated for cases of observed and expected low PChE activity at 20% reductions.31 The level of statistical significance was at P < 0.05.
3. RESULTS
The demographic data as well as the surgical operation characteristics (duration of operation, and time to recovery from anesthesia) of 102 participants who underwent elective CS are given in Table 1. The mean age in years of both GA and SA groups was (29.1 ± 5.5). The duration of surgery for Group GA was significantly longer than that of the Group SA, and the recovery time from the GA was substantially shorter than that of the SA.
| Table 1: Comparative demographic and surgical characteristics of both groups | |||
| Variable | Group GA
(n = 52) |
Group SA
(n = 50) |
P-value |
| Age (y) | 28.24 ± 5.55 | 29.98 ± 5.37 | 0.11147 |
| Weight (kg) | 74.55 ± 12.501 | 73.59 ± 12.90 | 0.848 |
| Height (cm) | 158.86 ± 5.78 | 160.37 ± 6.25 | 0.484 |
| Gestation week | 37.08 ± 1.14 | 37.73 ± 1.09 | 0.132 |
| Parity | 1.52 ± 1.53 | 2.06 ± 2.02 | 0.130 |
| Gravidity (frequency) | 2.56 ± 1.53 | 3.16 ± 2.07 | 0.097 |
| Duration of operation (min) | 30.29 ± 11.304 | 22.12 ± 8.65* | 0.0001 |
| Time to recovery from anesthesia (min) | 37.46 ± 11.16 | ** | |
| * Significantly different from the propofol group, Unpaired Student’s-t-test, p < 0.05.
**The time to recovery ranged between 4−6 h.; Values expressed as mean ± SD. |
|||
| Table 2: Comparison of plasma and erythrocyte cholinesterase (ChE) activities in both groups before GA and SA and after the operation | |||||
| Variable | Group GA
(n = 52) |
% Change | Group SA
(n = 50) |
% Change | P – value (GA; SA) |
| Plasma ChE (Δ pH/20min) | |||||
| Before | 0.75 ± 0.160 | –17 | 0.71 ± 0.168 | –11 | 7.0169E-06; 5.5731E-08 |
| After | 0.62 ± 0.217* | 0.63 ± 0.184* | |||
| Erythrocyte ChE (Δ pH/20min) | |||||
| Before | 1.48 ± 0.111 | –2 | 1.43 ± 0.276 | 0 | 0.241; 0.670 |
| After | 1.45 ± 0.172 | 1.43 ± 0.283 | |||
| Values are mean ± SD; *Significantly different from the corresponding pre-anesthetic value, P < 0.05. | |||||
| Table 3: Comparison of plasma malondialdehyde (MDA) and total antioxidant status (TAS) levels before anesthesia and after the surgery | |||||
| Variable | Group GA
(n=52) |
% Change | Group SA
(n= 50) |
% Change | P-value
(GA; SA) |
| MDA (µmol/L) | |||||
| Before | 4.13 ± 0.958 | –13 | 4.52 ± 1.052 | –24 | 8.2805E-05; 6.793E-12 |
| After | 3.58 ± 0.706* | 3.43 ± 0.827* | |||
| TAS (mmol Trolox Equivalent/L) | |||||
| Before | 1.29 ± 0.378 | +2 | 1.36 ± 0.265 | –1.5 | 0.649; 0.758 |
| After | 1.31 ± 0.353 | 1.34 ± 0.302 | |||
| Values are mean ± SD; *Significantly different from the corresponding pre-anesthetic value, P < 0.05. | |||||
3.1. Blood ChE activity
PChE activities before anesthesia and after the surgery in Group GA and Group SA were measured. In both groups, the PChE activity was significantly (P < 0.05) reduced after the CS in comparison to pre-anesthesia values by 17% and 11%, respectively. However, there was no significant difference in EChE activity in both groups with respect to their pre-anesthesia values (Table 2).
3.2. Plasma oxidative stress biomarkers
In both groups the plasma MDA level was significantly (P < 0.05) reduced after the operation in comparison to the pre-anesthesia values. MDA level reduced by 13% and 24%, in Group GA and Group SA, respectively. However, there was no significant difference in the TAS
| Table 4: The frequency of occurrence of reduced PChE activity (Δ pH/20min) at values ≤ 0.62 for GA and at ≤ 0.63 for SA | ||||
| Sample time | Group GA | Group SA | ||
| PChE ≤ 0.62* | PChE > 0.62 | PChE ≤ 0.63* | PChE > 0.63 | |
| After operation | 25 | 27 | 26 | 24 |
| Before anesthesia | 12 | 40 | 19 | 31 |
| *Significantly different bench mark point for PChE activity compared to the corresponding pre-anesthesia value as reported in Table 2. | ||||
| Table 5: Odds ratio and risk ratio of significantly reduced PChE activity in both groups | ||
| Statistics | Group GA | Group SA |
| Odds ratio | 3.09 | 1.77 |
| 95% confidence interval | 1.3,7.2 | 0.8,3.9 |
| p value | 0.009 | 0.161 |
| Risk ratio | 2.08 | 1.37 |
| 95% confidence interval | 1.2, 3.7 | 0.9, 2.1 |
| p value | 0.012 | 0.165 |
in both groups with respect to their pre-anesthesia values (Table 3).
3.3. Risks of reduced PChE after the CS
We constructed a table of odds ratio (Table 4) using the frequency of distribution of PChE activity values (Δ pH/20 min) ≤ 0.62 for the GA and ≤ 0.63 for SA in women who underwent CS. It was noticed that estimation of the odds ratio and the risk ratio indicated a risk factor of reduced PChE activity in women who underwent CS with the use the general anesthetic propofol (Table 5).
3.4. Correlation between PChE, EChE and MDA
There was no correlation between PChE vs. EChE (r = -0.086), PChE vs. MDA (r = 0.130) and EChE vs. MDA (r = -0.23) in women set undergoing elective CS and either GA or SA, taking into account the pre-anesthetic and after the operation ChE activities.
4. DISCUSSION
Current evidence strongly suggests an increasing preference of parturients for elective cesarean-delivery, apparently to avoid labor pains and the stress related to normal delivery, in many regions around the world,1−3,32,33 including Iraq.4,34 For elective CS, the most preferred GA is with propofol and SA with bupivacaine. The present study was conducted in four hospitals in Duhok, Iraq, to examine the effects of these two most commonly used anesthetics, on blood OS biomarker and ChE activity. The most prominent finding of the present study was a reduction in PChE activity post-operatively in both types of anesthesia. This enzyme is found in the liver, plasma and other tissues, and it is involved in the metabolism of some anesthetics and related muscle relaxants.35,36 Reduced PChE activity is a biomarker of exposure to pesticides,29,31,37 and it has been implicated in different cardiovascular and hemodynamic imbalances during anesthesia, especially at the level of the autonomic nervous system.9,24,38
The mean PChE activities (Δ pH/20min) before anesthesia were 0.75 and 0.71 in GS and SA, respectively. These values are generally below normal ranges reported by others,28,29 using the same electrometric method we applied in the present study. This could be related to physiological hemodynamic changes expected during pregnancy.35,39-42 Within this context, the results of the present study demonstrated that PChE is further reduced in both GA and SA groups after the CS, and therefore, caution should be practiced during application of anesthetics and neuromuscular blocking agents in surgical operations for the CS. Additionally, it is reasonable to suggest that monitoring blood ChE activity should be a routine integration of maternal health assessment before any CS.
The reduction in PChE activity after the CS in the GA group could be due to the direct effect of propofol. Propofol has been reported to interact with central cholinergic system, during GA, possibly by reducing acetylcholine release and cholinergic functions that are related to anesthesia-induced immobility, amnesia and loss of consciousness.43 In support of this notion,
physostigmine reverses propofol-induced unconsciousness in human volunteers,44 and its pretreatment increases the dose of propofol needed to induce unconsciousness in patients.45 On the other hand, the local anesthetic bupivacaine was reported to possess an inhibitory action over PChE activity.46 This effect could explain the reduction in PChE activity in the SA group of the present study.
Alterations in serum ChE activity were not attributed to the bupivacaine spinal application alone in patients undergoing lower limb surgery, and they were rather attributed in part to surgery-related depression in cellular secretory function as well as to protein depletion due to blood loss.24 It should be stressed here, that we cannot rule out the possible effects of adjuvants, maintenance anesthetics and other medications on PChE activity when used intra-operatively during the CS. Such a reduction of PChE activity after the anesthesia calls for careful hemodynamic support during CS.7,9,24,35,39,42
As reported earlier, EChE activity increases during pregnancy, and this was also true in the present pre-anesthetic activity of this enzyme when compared to normal values reported in non-pregnant women using the same electrometric method.28,29 However, in contrast to PChE, there was no significant difference between the pre-anesthesia and post-CS values of EChE activity in the present study, suggesting a differential effect of both propofol and bupivacaine on PChE vs. EChE. This finding is also in accordance with a previous report about the selective inhibition of butyryl ChE (vs. true ChE) by bupivacaine.47 However, in the study of Gramavy,22 there was a significant reduction of EChE activity with propofol in non-pregnant women as well as in males. This discrepancy could be related to the fact that in the present study the women were in the last stage of pregnancy and were very close to the due date of delivery, which is known to be accompanied by various metabolic changes, including activities of PChE and EChE.39,40,48
Pregnant women who undergo CS are at high risk of suffering from OS due to the physical and emotional stress of surgery and the anesthesia.19 In the present study, pre-anesthesia (GA) levels of plasma MDA significantly decreased after the CS, suggesting a reduction in the OS status of the women.
Several factors may have contributed to the present finding; most importantly, propofol, being an antioxidant, reduces the impact of OS, probably through inhibition of nuclear factor kappa B (NF-κB) signal transduction pathway in liver.49 The plasma OS biomarker MDA, is a byproduct of lipid peroxidation of cell membranes, which also occurs during pregnancy.50,51
It has been reported that propofol results in inhibition of lipid peroxidation and enhancement of antioxidant capacity.49,52 The antioxidant effect of propofol could be due to the phenolic structure of the drug which is similar to α-tocopherol, an antioxidant.53 While there was no significant effect at the TAS level with propofol in the present study, the results suggest a specific effect of the anesthetic at the level of MDA mechanism in pregnant women who underwent CS. However, TAS is the result of overall contributions of several antioxidant mechanisms such as albumin, iron, bilirubin, uric acid;54,55 and it is possible that all these variables were not affected considerably enough by the present CS/anesthetic paradigm.
Similar to the effect of propofol (reduction of the plasma MDA level) in the present study, SA with bupivacaine also significantly reduced MDA level, but not that of TAS after the CS. This finding is reported at present as the first of its kind, though its mechanism is not yet clear. However, it is conceivable to deduce that removing the burden of pregnancy by the CS might have contributed to the antioxidant properties of both propofol and bupivacaine. This effect, however, needs further follow up clinically.
There was no correlation between the PChE and EChE activity, as well as between PChE activity and MDA level, although the PChE activity and MDA values were affected by anesthetics in the present study. This was also true about EChE activity and MDA level. This finding correlates with that of Garmavy22 who found also poor correlation (r = 0.323) between PChE and EChE activity in patients who received propofol. In contrast, a study in pregnant women with gestational diabetes reported a negative correlation between serum ChE activity and the MDA level and a strong correlation between this enzyme and total antioxidant activity in the serum;56 apparently serum ChE acted as a scavenger of free radicals in the presence of OS.
5. LIMITATIONS
Extended follow up and interval blood sampling was needed; however, none of the participants were willing to pursue the study beyond the CS. The choices of anesthetics (general or local) were limited in the hospitals, probably because of their availability and the preferences of the surgeons. Otherwise, it would have been interesting to study other anesthetics that can be applied in the CS.
6. CONCLUSION
Propofol and bupivacaine were used for general anesthesia and spinal anesthesia respectively, for elective cesarean sections in Duhok, Iraq. Inhibition of plasma ChE activity and reduced oxidative stress biomarker MDA occurred differentially after the cesarean sections under GA and SA; with the possibility of health risk association with reduced plasma ChE in case of using propofol. Clinical follow-up studies on the variables used are warranted on women after the cesarean sections.
7. Data availability
The numerical data of this study is available with the first author.
8. Conflict of interests
The authors declare no conflicts of interest with respect to the authorship and/or publication of this article.
9. Acknowledgments
This study was supported by the University of Duhok.
10. Authors’ contribution
RSK: Dealt with the participants, obtained blood samples and executed laboratory assays; conducted literature search, statistical analyses and shared in drafting the manuscript.
FKM: Conceptualized and supervised the study, shared in literature search, statistical analyses and drafting the manuscript. Both authors have read the manuscript and approve it for publication.
11. REFERENCES
- Al-Husban N, Elmuhtaseb MS, Al-Husban H, Nabhan M, Abuhalaweh H, Alkhatib YM, et al. Anesthesia for cesarean section: retrospective comparative study. Int J Womens Health. 2021;13:141−52. [PubMed] DOI: 2147/IJWH.S292434.
- Lonnée HA, Madzimbamuto F, Erlandsen ORM, Vassenden A, Chikumba E, Dimba R, et al. Anesthesia for cesarean delivery: a cross-sectional survey of provincial, district, and mission hospitals in Zimbabwe. Anesth Analg. 2018;126(6):2056−64. [PubMed] DOI: 1213/ANE.0000000000002733.
- Iddrisu M, Khan ZH. Anesthesia for cesarean delivery: general or regional anesthesia—a systematic review. Ain-Shams J Anesthesiol. 2021;13:1. DOI: 1186/s42077-020-00121-7
- Karam RS, Mohammad FK. The use of anesthetics for cesarean section delivery in women in Duhok, Kurdistan region, Iraq. J Ideas Health. 2022;5(4):755−59. [FreeFullText]
- Kim WH, Hur M, Park SK, Yoo S, Lim T, Yoon HK, et al. Comparison between general, spinal, epidural, and combined spinal-epidural anesthesia for cesarean delivery: a network meta-analysis. Int J Obstet Anesth. 2019;37:5−15. [PubMed] DOI: 1016/j.ijoa.2018.09.012
- Kepekçi AB. Choice of anesthesia method in cesarean delivery: communication between anesthesiologist and obstetrician. J Contemp Med. 2019;9(1):27−31. DOI: 16899/gopctd.512719
- Davis L, Britten JJ, Morgan M. Cholinesterase. Its significance in anaesthetic practice. 1997;52:244–60. [PubMed] DOI: 10.1111/j.1365-2044.1997.084-az0080.x
- Kundović SA, Rašić D, Popović L, Peraica M, Črnjar K. Oxidative stress under general intravenous and inhalation anaesthesia. Arh Hig Rada Toksikol. 2020;71(3):169−77. [PubMed] DOI: 2478/aiht-2020-71-3437
- Brzezinski-Sinai Y, Zwang E, Plotnikova E, Halizov E, Shapira I, Zeltser D, et al. Cholinesterase activity in serum during general anesthesia in patients with or without vascular disease. Sci Rep. 2021;11(1):16687. [PubMed] DOI: 1038/s41598-021-96251-5
- Khoshraftar E, Ranjbar A, Kharkhane B, Tavakol Heidary S, Gharebaghi Z, Zadkhosh N. Antioxidative effects of propofol vs. ketamin in individuals undergoing surgery. Arch Iran Med. 2014;17(7):486−9. [PubMed]
- Senoner T, Velik-Salchner C, Luckner G, Tauber H. Anesthesia-induced oxidative stress: are there differences between intravenous and inhaled anesthetics? Oxid Med Cell Longev. 2021;2021:8782387. [PubMed] DOI: 1155/2021/8782387
- Aroosa S, Sattar A, Javeed A, Usman M, Hafeez MA, Ahmad M. Protective effects of dexmedetomidine infusion on genotoxic potential of isoflurane in patients undergoing emergency surgery. Int J Clin Pract. 2023;2023:7414655. [PubMed] DOI: 1155/2023/7414655
- Ulugol H, Aksu U, Kocyigit M, Kilercik M, Karduz G, Okten M, et al. Comparative effects of blood and crystalloid cardioplegia on cellular injury and oxidative stress in cardiovascular surgery. Ann Thorac Cardiovasc Surg. 2019;25(1):10−7. [PubMed] DOI: 5761/atcs.oa.18-00113
- Hol JW. The biochemical impact of surgery and anesthesia. Erasmus University Rotterdam; 2014. Available from: http://hdl.handle.net/1765/77645
- Dai Y, Liu J, Yuan E, Li Y, Wang Q, Jia L, et al. Gestational age-specific reference intervals for 15 biochemical measurands during normal pregnancy in China. Ann Clin Biochem. 2018;55(4):446−52. [PubMed] DOI: 1177/0004563217738801
- Chen Y, Liu W, Gong X, Cheng Q. Comparison of effects of general anesthesia and combined spinal/epidural anesthesia for cesarean delivery on umbilical cord blood gas values: a double-blind, randomized, controlled study. Med Sci Monit. 2019;25:5272−9. [PubMed] DOI: 12659/MSM.914160
- Chiarello DI, Abad C, Rojas D, Toledo F, Vázquez CM, Mate A, et al. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim Biophys Acta Mol Basis Dis. 2020;1866(2):165354. [PubMed] DOI: 1016/j.bbadis.2018.12.005
- Kutlesic MS, Kocic G, Kutlesic RM. [The effects of remifentanil used during cesarean section on oxidative stress markers in correlation with maternal hemodynamics and neonatal outcome: a randomized controlled trial]. Braz J Anesthesiol. 2019;69(6):537−45. [PubMed] DOI: 1016/j.bjan.2019.05.005
- Akin F, Kozanhan B, Deniz CD, Sahin O, Goktepe H, Neselioglu S, et al. Effects of the anesthesia technique used during cesarean section on maternal-neonatal thiol disulfide homeostasis. Minerva Anestesiol. 2019;85(11):1175−83. [PubMed] DOI: 23736/S0375-9393.19.13598-5
- Zhang Z, Yan B, Li Y, Yang S, Li J. Propofol inhibits oxidative stress injury through the glycogen synthase kinase 3 beta/nuclear factor erythroid 2-related factor 2/heme oxygenase-1 signaling pathway. Bioengineered. 2022;13(1):1612−25. [PubMed] DOI: 1080/21655979.2021.2021062
- Li X, Xiang H, Zhang W, Peng C. The effects of remifentanil combined with propofol on the oxidative damage and the stress and inflammatory responses in cardiac surgery patients. Am J Transl Res. 2021;13(5):4796−803. [PubMed]
- Garmavy HM. General anesthesia and oxidative stress status: interaction with cholinesterase inhibitors [dissertation]. Duhok, Iraq: University of Duhok; 2009.
- Meuret P, Backman SB, Bonhomme V, Plourde G, Fiset P. Physostigmine reverses propofol-induced unconsciousness and attenuation of the auditory steady state response and bispectral index in human volunteers. Anesthesiology. 2000;93(3):708−17. [PubMed] DOI: 1097/00000542-200009000-00020
- Kluge WH, Kluge HH, König U, Venbrocks RA, Bauer HI, Lange M. Effect of bupivacaine application on cholinesterase activities, total protein- and albumin concentration in serum and cerebrospinal fluid. Scand J Clin Lab Invest. 2002;62(7):495−502. [PubMed] DOI: 1080/003655102321004503
- Sung TY, Jee YS, You HJ, Cho CK. Comparison of the effect of general and spinal anesthesia for elective cesarean section on maternal and fetal outcomes: a retrospective cohort study. Anesth Pain Med (Seoul). 2021;16(1):49−55. [PubMed] DOI: 17085/apm.20072
- Hayata E, Nakata M, Takano M, Nagasaki S, Oji A, Sakuma J, et al. Biochemical effects of intraoperative cell salvage and autotransfusion during cesarean section: a prospective pilot study. J Obstet Gynaecol Res. 2021;47(5):1743−50. [PubMed] DOI: 1111/jog.14738
- Solangi SA, Siddiqui SM, Khaskheli MS, Siddiqui Comparison of the effects of general vs spinal anesthesia on neonatal outcome. Anaesth. pain intensive care 2012;16(1):18-23 [Free full text]
- Mohammad FK, Alias AS, Ahmed OA. Electrometric measurement of plasma, erythrocyte, and whole blood cholinesterase activities in healthy human volunteers. J Med Toxicol. 2007;3(1):25-30. [PubMed] DOI: 1007/BF03161035
- Garmavy HMS, Mohammed AA, Rashid HM, Mohammad FK. A meta-analysis of normal human blood cholinesterase activities determined by a modified electrometric method. J Med Life. 2023;16(1):22-34. [PubMed] DOI: 25122/jml-2022-0215
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-10. [PubMed] DOI: 1016/s0076-6879(78)52032-6
- Odisho SK, Mohammad FK. Blood cholinesterase activities and oxidative stress status among farmworkers using pesticides in Duhok, KRG, Iraq. J Ideas Health. 2022;5(4):786-93. [FreeFullText]
- Ghaffari S, Dehghanpisheh L, Tavakkoli F, Mahmoudi H. The effect of spinal versus general anesthesia on quality of life in women undergoing cesarean delivery on maternal request. Cureus. 2018;10(12):e3715. [PubMed] DOI: 7759/cureus.3715
- Ilska M, Kołodziej-Zaleska A, Banaś-Fiebrich E, Brandt-Salmeri A, Janowska-Tyc E, Łyszczarz A, et al. Health-related quality-of-life among pregnant women after first, second, and multiple cesarean sections in the perinatal period: a short-term longitudinal study. Int J Environ Res Public Health. 2022;19(24):16747. [PubMed] DOI: 3390/ijerph192416747
- Abdulkader, MA, Mohammed, HN, Salih, HM. Anesthetic for cesarean section: the current practice in Kurdistan region-Iraq. Med J Babylon. 2017;14(1):8−19. [FreeFullText]
- Andersson ML, Møller AM, Wildgaard K. Butyrylcholinesterase deficiency and its clinical importance in anaesthesia: a systematic review. Anaesthesia. 2019;74(4):518−28. [PubMed] DOI: 1111/anae.14545
- Zhang C, Cao H, Wan ZG, Wang J. Prolonged neuromuscular block associated with cholinesterase deficiency. Medicine (Baltimore). 2018 Dec;97(52):e13714. [PubMed] DOI: 1097/MD.0000000000013714
- Samareh A, Asadikaram G, MojtabaAbbasi-Jorjandi, Abdollahdokht D, Abolhassani M, Khanjani N, et al. Occupational exposure to pesticides in farmworkers and the oxidative markers. Toxicol Ind Health. 2022;38(8):455−69. [PubMed] DOI: 1177/07482337221106754
- Ofek K, Soreq H. Cholinergic involvement and manipulation approaches in multiple system disorders. Chem Biol Interact. 2013;203:113–9. [PubMed] DOI: 1016/j.cbi.2012.07.007
- de Peyster A, Willis WO, Liebhaber M. Cholinesterase activity in pregnant women and newborns. J Toxicol Clin Toxicol. 1994;32(6):683−96. [PubMed] DOI: 3109/15563659409017975
- Venkataraman BV, Iyer GY, Narayanan R, Joseph T. Erythrocyte and plasma cholinesterase activity in normal pregnancy. Indian J Physiol Pharmacol. 1990;34(1):26−8. [PubMed]
- Elton RJ. Pregnancy-induced cholinesterase deficiency. Anaesthesia. 1999 Apr;54(4):398. [PubMed] DOI: 1046/j.1365-2044.1999.00867.x
- Davies P, Landy M. Suxamethonium and mivacurium sensitivity from pregnancy-induced plasma cholinesterase deficiency. Anaesthesia. 1998;53(11):1109−11. [PubMed] DOI: 1046/j.1365-2044.1998.00581.x
- Leung LS, Luo T. Cholinergic modulation of general anesthesia. Curr Neuropharmacol. 2021;19(11):1925−36. [PubMed] DOI: 2174/1570159X19666210421095504
- Meuret P, Backman SB, Bonhomme V, Plourde G, Fiset P. Physostigmine reverses propofol-induced unconsciousness and attenuation of the auditory steady state response and bispectral index in human volunteers. Anesthesiology. 2000 Sep;93(3):708−17. [PubMed] DOI: 1097/00000542-200009000-00020
- Fassoulaki A, Sarantopoulos C, Derveniotis C. Physostigmine increases the dose of propofol required to induce anaesthesia. Can J Anaesth. 1997;44(11):1148−51. [PubMed] DOI: 1007/BF03013335
- Galenko-Yaroshevskii AP, Derlugov LP, Ponomarev VV, Dukhanin AS. Pharmacokinetics and pharmacodynamics of a new local anesthetic agent. Bull Exp Biol Med. 2003;136(2):170−3. [PubMed] DOI: 1023/a:1026323124831
- Kluge WH, Kluge HH, Bauer HI, Pietsch S, Anders J, Venbrocks RA. Acetylcholinesterase assay for cerebrospinal fluid using bupivacaine to inhibit butyrylcholinesterase. BMC Biochem. 2001;2:17. [PubMed] DOI: 1186/1471-2091-2-17
- Evans RT, Wroe JM. Plasma cholinesterase changes during pregnancy. Their interpretation as a cause of suxamethonium-induced apnoea. Anaesthesia. 1980;35(7):651−4. [PubMed] DOI: 1111/j.1365-2044.1980.tb03878.x
- Brasil LJ, San-Miguel B, Kretzmann NA, Amaral JL, Zettler CG, Marroni N, et al. Halothane induces oxidative stress and NF-kappaB activation in rat liver: protective effect of propofol. Toxicology. 2006;227(1−2):53−61. [PubMed] DOI: 1016/j.tox.2006.07.013
- Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem. 2017;524:13−30. [PubMed] DOI: 1016/j.ab.2016.10.021
- de Lucca L, Jantsch LB, Vendrame SA, de Paula HL, Dos Santos Stein C, Gallarreta FMP, et al. Variation of the oxidative profile in pregnant women with and without gestational complications. Matern Child Health J. 2022;26(10):2155−68. [PubMed] DOI: 1007/s10995-022-03475-6
- Li Volti G, Basile F, Murabito P, Galvano F, Di Giacomo C, Gazzolo D, et al. Antioxidant properties of anesthetics: the biochemist, the surgeon and the anesthetist. Clin Ter. 2008;159(6):463−9. [PubMed]
- Braz MG, Braz LG, Freire CMM, Lucio LMC, Braz JRC, Tang G, et al. Isoflurane and propofol contribute to increasing the antioxidant status of patients during minor elective surgery: a randomized clinical study. Medicine (Baltimore). 2015;94(31):e1266. [PubMed] DOI: 1097/MD.0000000000001266
- Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37(2):112−9. [PubMed] DOI: 1016/j.clinbiochem.2003.10.014
- Bozkaya G, Karaca I, Fenercioglu O, Yildirim Karaca S, Bilgili S, Uzuncan N. Evaluation of maternal serum ischemia modified albumin and total antioxidant status in ectopic pregnancy. J Matern Fetal Neonatal Med. 2019;32(12):2003−8. [PubMed] DOI: 1080/14767058.2017.1422718
- Omu AE, Al-Azemi MK, Omu FE, Fatinikun T, Abraham S, George S, et al. Butyrylcholinesterase activity in women with diabetes mellitus in pregnancy: correlation with antioxidant activity. J Obstet Gynaecol. 2010;30(2):122−6. [PubMed] DOI: 3109/01443610903443913