Ahmed Haddadi1, Mohamed Lemdani2, Karim Laribi3
1Research Unit EA 2694, Public Health Epidemiology & Quality of Care, Medical University, Lille2, (France) 2Research Unit EA 2694, Public Health Epidemiology & Quality of Care, Biostatistics Laboratory, Faculty of Pharmacy ; University Lille2, (France)
3France Accreditation, University Paris Descartes, (France)
Correspondence: Ahmed Haddadi, Research Unit EA 2694, Public Health Epidemiology & Quality of Care, Medical University, Lille2, (France); Tel: +33 6 98 91 16 69; E-mail: ahmed.haddadi@univ-lille2.fr
SUMMARY
The hypothalamic-pituitary-adrenal axis has a great importance in the process of the body's fight against any infection.
In fact, several studies, including reports of an experts’ conference and those of a consensus conference, reveal a high probability of the alteration of the endocrine response in a context of septic shock.
This alteration might appear as ACTH deficiency (adrenocorticotropic deficiency) which pejorative prognosis is attested even if the pathophysiological mechanisms of this inappropriate endocrine response remain poorly understood.
This inappropriate endocrine response might represent thus a rational which is pertinent enough for the use of corticosteroids in the treatment of the septic shock. This rational is enhanced even more on the one hand by a better understanding of the corticosteroids action mechanisms process. On the other hand, by good proved benefit-risk ratio, of the use of corticosteroids, in a given situation dominated by an uncontrolled systemic inflammatory reaction, such as the septic shock.
According to the results of several studies –animal and clinical – the doses of 200 mg /day of hydrocortisone (or equivalent) might allow to restore in a substantial way the hemodynamic and immunizing homeostasis during the severe sepsis and the septic shock.1
A systematic review of randomized trials shows that a corticosteroid therapy at low-dose (200 to 300mg / day of hydrocortisone or equivalent) administered over a long period (at least 5 days of full dose) would improve the 28-day survival at hospital, it would also increase the probability of the shock resolution, it will decrease the period of stay in the intensive care unit and it wouldn’t cause any serious side effects with the exception of metabolic complications.1
However, to date, despite all these provided elements in current practice, the adoption of corticosteroids treatment in septic shock remains very controversial.
This controversy is widely based on the results of some studies that do not only show the absence of any benefit -linked to the use of corticosteroids - but also the possibility of the prognostic aggravation.
Key words: Endocrine; Corticosteroids; Septic shock; Sepsis; Cortisol; Glucocorticoid; Adrenocorticotropic hormone; Inflammatory cytokines
Citation: Haddadi A, Lemdani M, Laribi K. Efficacy of corticosteroids for the treatment of septic shock. Anaesth Pain & Intensive Care 2016;20(4):488-502
Received: 23 Sep 2016; Reviewed: 23 Nov 2016; Corrected: 7 Dec 2016; Accepted: 12 Dec 2016
INTRODUCTION
The physiological functions of cortisol were objectified in the 1930s, and the use of corticosteroids in the treatment of severe infections was mentioned in 1940.1, 2-5
Since then, corticosteroids have proven efficiency in the treatment of some infections.1, 2-4
This is through benefit contribution in survival terms (bacterial meningitis, tuberculosis meningitis, tuberculosis pericarditis, severe typhoid fever, tetanus, moderate to severe pneumocystis) or by long-term benefits (bacterial arthritis). Or finally, over a symptomatically benefit (herpes zoster, infectious mononucleosis, croup, pharyngitis, phlegmon, cellulitis, chronic otitis media, glandular tuberculosis, pleural tuberculosis).1
However, in the frame of severe sepsis and septic shock, their use remains very controversial.
In fact, recent consensus declarations as well as the results of some meta-analyses have reconsidered the recommendations given by Minneci et al. The said recommendations mentioned that a low dose of steroid treatment should be considered almost systematically for every patient suffering from a vasopressor dependent septic shock.6, 7-11
This controversy in the points of view is mainly due to the absence of current tangible clinical proofs or even the ones in progress and to the insufficient multiple meta-analysis results in the frame of answering the following questions: Is a corticosteroid therapy really beneficial for patients suffering from severe sepsis and septic shock, What is the best dose recommended, Should the treatment be intermittent or continuous, Should its interruption be brutal or based on a decreasing process, Should the treatment duration be fixed or should it depend on the clinical response.
Thus, the aim of this study tackles, after the explanation of the concept of the relative adrenal insufficiency associated with the septic shock, a systematic analysis of the randomized trials over the substitutive opotherapy in septic shock and severe sepsis.
CORTISOL PHYSIOLOGY
Cortisol is the main glucocorticoid. It is a steroid hormone composed of 19 carbon atoms synthesized from cholesterol by the cytochrome enzyme P450. It is secreted by the zona fasciculata, which is the deepest adrenal corticosteroid area.
It circulates in the plasma as an active free form (5-10% of total cortisol), or as an inactive form reversibly linked mainly to the following two binding proteins: cortisol binding protein (CBG), and albumin.13
Its penetration into cells is passive; it binds with a specific cytosolic soluble receptor, glucocorticoid receptor (GR) Type II.
This connection, characterized by the cortisol and its receptor, activates the complex cortisol-GR by a process involving dissociation of the proteins known as chaperones, especially those of heat shock 90 and 70 and FK-506 binding proteins).14
Thus, the complex cortisol-GR can migrate actively (ATP dependent) in the nucleus where it plays the transcription factor role.
It interacts with several specific DNA sites located in the region promoting target genes, activating several genes transcription.
Nevertheless, the complex cortisol-GR can also interact at the cell membrane level or with different cytosolic proteins especially with other transcription factors, influencing the activity of the latter on their own target genes.
The cortisol is metabolized in the liver and the kidney.15 It is worth mentioning that it is transformed into inactive metabolite, cortisone, by the enzyme 11-β-HSD2. However, due to 11-β-HSD1, several tissues, including the liver, the adipose tissue, and the bone tissue have the ability to convert the inactive cortisone to active cortisol.16
REGULATING ROLE OF THE HYPOTHALAMUS-PITUITARY AXIS
The adrenocorticotropic hormone (ACTH), produced by the anterior pituitary gland stimulates largely the cortisol synthesis and secretion.17
However, the secretory regulation of ACTH depends on several factors, including the corticotropin-releasing hormone (CRH), which is secreted by the hypothalamus and liberated in the hypothalamus-pituitary axis.
In this intricate scheme and interdependence, we will notice that arginine vasopressin (AVP) stimulates weakly ACTH secretion, but it increases the CRH action. Besides, catecholamines, angiotensin II, serotonin and vasoactive intestinal peptide (VIP) are stimulating elements of the ACTH secretion.
As for ACTH secretion, some inflammatory cytokines such as interleukin IL-1, IL-2, IL-6, tumor necrosis factor (TNF) are naturally stimulating. However, other cytokines such as the transforming growth factor are inhibitors.
As regards the CRH, its production is stimulated by adrenergic agonists (noradrenalin), serotonin and inhibited by the substance P, the opiates and the γ-aminobutyric acid.
Inflammatory cytokines (IL-1, IL-2, IL-6, and TNF) influence the CRH production.
Finally, on the one hand, it is worth mentioning that there is a negative feedback of glucocorticoids on the corticotropic axis which is revealed as an inhibition of the production of ACTH, CRH and AVP.
On the other hand, the secretion of the corticotropic axis hormones is pulsating during the circadian cycle reaching its maximum in the morning between 6 am and 8 am, then it decreases rapidly until noon, afterwards it registers a slower decrease until midnight.18
GLUCOCORTICOIDS PHYSIOLOGICAL EFFECTS ON THE MAIN ORGANS AND FUNCTIONS
Metabolic effects
The glucocorticoids are highly involved in glucose metabolism. In the liver, they stimulate the gluconeogenesis and the glycogenolysis. By increasing peripheral resistance to insulin, they inhibit the glucose cell extraction leading thus to the increase of the blood glucose.19 Beyond stimulating the glucagon and adrenaline secretion, they also affect lipid metabolism, they stimulate the lipolysis and decrease the glucose consumption by adipocytes.
It is worth mentioning, however, that cortisol inhibits the protein synthesis, it activates the muscle protein breakdown liberating thus amino acids serving as substrate for gluconeogenesis. At the bone level, the glucocorticoid action on calcium metabolism is acknowledged.
Furthermore, cortisol activates the osteoclasts, inhibits the osteoblasts, inhibits the intestinal absorption of calcium, and increases the urinary secretion of calcium by decreasing renal re-absorption.
Immunological and anti-inflammatory effects
The immunomodulator and anti-inflammatory effects of cortisol have been widely documented. However, to date, they are still weakly clarified.20
Nevertheless, it is proved that cortisol acts almost over the entire cellular panoply of immunity (poly-nuclear neutrophils, lymphocytes, monocytes, macrophages, eosinophils, basophils), by affecting important cellular functions, such as migration and chemotaxis, apoptosis, phagocytosis, anti-oxidative metabolism, adhesion and communication (cytokine production).
It promotes the lymphocytes migration starting the circulation towards lymphoid organs. It is also behind the migration of neutrophils and macrophages inhibition towards inflammation sites, which leads to the decrease of local inflammation.
Even though glucocorticoids have a stimulating action of eosinophil apoptosis, they are originally protectors of monocyte apoptosis.
In humoral immunity, cortisol has a definite role as a modulator since it inhibits the IL-12 production by the macrophages and monocytes, which affects de facto the lymphocyte differentiation by acting on the balance of Th1-Th2 in favor of Th2 cells. The IL-4 secretion (which is normally inhibited by IL-12) is then increased.
The promotion of Th2 activity and humoral immunity is joined to the suppression of cellular immunity, noting that Th1 and Th2 activities are mutually inhibitory. However, all these observations objectified in vitro must be considered with some reservation waiting to be confirmed in vivo.
At the cellular level, the inflammatory response is modulated by cortisol.
In fact, even though glucocorticoids slow down the synthesis or the action of the majority of pro-inflammatory cytokines (IL-1, IL-2, IL-3, IL-6, IFN, TNF), of chemokines, eicosanoids, bradykinin, and MIF, they stimulate the production of many anti-inflammatory factors such as the receptor agonist to IL-1, soluble TNF receptor, IL-10 and the transforming growth factor β.
This anti-inflammatory action is consolidated by inhibiting cyclooxygenase production and NO synthase induced form, which are inflammation key enzymes.
Finally, it has been demonstrated that cortisol has an action on over nearly 2,000 genes involved in immune response.21
Cardiovascular effects
Cortisol action on the kidney and vascular endothelium has an essential role in cardiovascular homeostasis.22 In fact, its action takes part in preserving the vascular tension, the vascular permeability and the total distribution of H2O volume in the vascular compartment. Its action mechanisms on the cardiovascular system remain, to date, poorly understood, but nonetheless they seem to be independent of mineral corticoids effects and of sympathetic system.23,24
In the smooth muscle, cortisol increases sensitivity to vasoconstrictor agents such as catecholamines and angiotensin II, especially by increasing the transcription and expression of receptors to these hormones.25
The cortisol effects on nitric oxide are complex; it increases the synthesis of the endothelial form of the nitric oxide synthase, maintaining thus the tissue perfusion.26 The glucocorticoids effects on the vasomotor tone are probably premature (within minutes) and they have a non-genomic mechanism.
Glucocorticoids molecular action mechanisms
The molecular action process of glucocorticoids is complex and remains, to date, not completely clarified.17,20,21 However, separate effects should be mentioned: non-genomic effects and genomic effects. Non-genomic effects are premature, occurring a few minutes after corticosteroids administration. They are either induced directly by interaction with membrane sites or linked to the release of chaperone proteins during the formation of glucocorticoids complex- GR. The first genomic effects are rather of late appearance. They require several hours of exposure to a glucocorticoid. In this case, the involved phenomenon is that of transrepression by sequestering in the cytoplasm the nuclear transcription factors such as NF- kB and AP-1, avoiding the transcription of genes for almost all the pro-inflammatory mediators.27,28
In addition, the NF-kB is normally kept as an inactive form in the cytoplasm by interaction with inhibitory proteins (IkB). However, the NF-kB / IkB complex is activated by phosphorylation and IkB protein degradation.
When the NF-KB is released, it migrates into the nucleus and binds to the promoter regions of target genes in order to initiate the transcription of multiple cytokines (tumor necrosis factor α, IL-1, IL-6), adhesion cells (intracellular adhesion molecul-1, E-selectin) and other inflammatory mediators.
The role of glucocorticoids is also the stimulation of IkB transcription.
Thus, the prominent role of the transactivation mechanisms in modulating the immune response by corticosteroids was also recently demonstrated.21
It is worth mentioning that the genomic transactivation represents a notably delayed response, occurring few days after the glucocorticoids administration (but some genes are also trans-activated in the first hours such as annexin-1, 2 adrenergic receptors and protein phosphatase 2).
The glucocorticoid-GR complex acts then as a transcription factor: it migrates into the nucleus and binds to target genes activating their transcription. Immune cells, such as monocytes, are then reprogrammed with cellular function modulation such as apoptosis, adhesion, cell motility, chemotaxis, phagocytosis, anti-oxidative reactions.
CORTICOSTEROID INSUFFICIENCY IN SEPTIC SHOCK
It is confirmed that in order to respond normally to a stress resulting from any type of serious acute pathology, the plasma cortisol has to be increased. It aims at maintaining the homeostatic balance.17,29
The processes brought into this hypercortisolemia are:
1-The increase of cortisol synthesis;
2- The increase of the conversion of cortisone into cortisol;
3- The decrease of the cortisol clearance;
4- The increase of free cortisol.
During stress, the production of cortisol is incidentally subordinated to the production of CRH and ACTH. Pro-inflammatory cytokines and the sympathetic nervous system become thus the important modulators of the corticotropic axis.29
Then, cortisol is synthesized in a continuous way instead of a pulsatile way, registering thus a loss of circadian rhythm and a decrease of the negative feedback capacity of cortisol on the hypothalamic-pituitary hormones.
However, the conversion of the cortisone into cortisol (catalyzed by 11- β -HSD1) is increased by the stromal cells such as fibroblasts.16
In case a renal and hepatic blood flow reduction is registered, then the cortisol clearance by the liver and the kidney might be pejoratively affected.15 This situation may also lead to increase the free cortisol concentrations and to rapidly decrease the CBG (Corticosteroid-binding globulin) and albumin concentrations.
In addition, it is important to note that in a stress context, the total rate of cortisol measured in the serum does not necessarily reflect that of the active free cortisol. The latter is probably underestimated.30
Animal and clinical studies suggest that sepsis may alter cortisol metabolism.31
On the contrary, pejorative and harmful implication of the corticosteroid insufficiency in sepsis towards the septic shock and death is quite probable.32,33
We have to fear the appearance of suprarenal insufficiency during the sepsis when the patients suffer from a hypothalamic-pituitary axis (e.g. Addison's disease) and who have been undergoing a corticosteroid therapy for more than seven days, regardless of the galenic form, as well as the patients diagnosed with sepsis and who have been exposed to treatments blocking the cortisol adrenal synthesis.34
A weak inappropriate production of cortisol synthesis (primary or secondary adrenal insufficiency) as well as an increased tissue resistance to glucocorticoids is probable.35
However, we have several processes of corticosteroid insufficiency in severe sepsis and septic shock which remain unclear to date.
The etiological cause of adrenal insufficiency (decrease of cortisol synthesis) in septic shock might be of pituitary origin (or rarely hypothalamic) when it is secondary. It is then characterized by an increase of ACTH production (or CRH production).
It might be of adrenal origin when it is primary. In this case, this type of insufficiency might follow the necrosis or hypothalamus hemorrhage or pituitary gland hemorrhage (if there is prolonged hypotension or severe coagulation disorders). It might follow as well a chronic or a latent secondary adrenal insufficiency degradation (hypothalamic or pituitary tumor, chronic congenital inflammation), or iatrogenic factors (corticosteroids therapy, opiates...).
The hemorrhage or the adrenal bilateral necrosis, the viral inflammation (HIV) or fungal inflammation, and the enzymatic cascade change that converts cholesterol to cortisol (e.g. etomidate) are also the pertinent causes of primary adrenal insufficiency.
However, there is also probably a tissue resistance to glucocorticoids, following a quantitative or qualitative alteration of the glucocorticoids receptors (this argument lacks evidence in vivo), through an increased activity of 11-β-hydroxysteroid dehydrogenase, an abnormal conversion of cortisol into inactive cortisone, an alteration of the cortisol transportation phenomena both at the systemic level (decrease in circulating CBG and albumin), the tissue level (abnormal cleavage of CBG-cortisol complex caused by elastase’s defect) and the cellular level (cortisol rejection outside the cell by proteins of "cleansing").
DIAGNOSIS
It is still hard to establish the diagnosis of the confirmed adrenal insufficiency during the sepsis for ICU patients. This is due to the non-specificity of clinical signs (fever, nausea, vomiting, abdominal pain, consciousness disorders, and hypotension) and biological signs (hypoglycemia, hyponatremia, hyper eosinophilia).34
As for the static hormones dosages do not allow to confirm the diagnosis with the exception of rare cases where the cortisol concentration is < 3 μg/dL.
It is important to highlight the limits of the total plasma cortisol concentration because it does not always reflect the free cortisol (especially in the case of hypoalbuminemia and during severe sepsis).30 Even when high levels of cortisol during sepsis are objectified it cannot be by itself a decisive diagnosis element neither a sign of a good adrenal function. It should suggest possible consequence of a defect elimination or peripheral resistance,15 hence the interest of a dynamic test to evaluate adrenal function.34
The traditional evaluation method used in intensive care units is the ACTH stimulation test, through the administration of 1 μg (low-dose short test) or 250 μg (conventional-dose) of cosyntropin.
However, the sensitivity and the specificity of this diagnostic test are debatable.34,36 The sensitivity of the synacthen test during the septic shock would be 68% with a specificity of 65%.5 Several experimental studies.29] have also demonstrated the reproducibility absence of the dynamic test in stress situations for the same person and the great variability in dosage techniques. Some authors have set then a total threshold value of cortisol in the case of a physiological stimulation (e.g. state of shock) or after stimulation by ACTH (low or high dose).36
The studies of Salgado and all have evaluated several diagnosis approaches of the alteration of adrenal function where ICU patients suffer from a septic shock. These studies revealed that an increase of cortisol total levels ≤ 9 μg / dL compared to the value at start, 60 min after a standard stimulation test at a dose of ACTH 250 μg might be the best prognostic criterion of the corticotropic insufficiency.36 Based on this diagnosis criterion, among the 102 patients suffering from septic shock and tested in the study of Salgado et al, 22.5% presented an adrenal dysfunction.
Another clinical trial that compares the ACTH stimulation at low dose vs. a standard dose - in the frame of the evaluation of the adrenal function- reveals that the absence of response to low-dose stimulation (1 μg of ACTH) might be correlated to a poor survival rate. For some of these patients with the absence of response, the standard dose test would not be of best interest.37
It is, however, important to mention that the ACTH stimulation tests only the response of the adrenal glands and does not evaluate the HPA axis.27
However, despite the persistent debate on diagnostic modalities of insufficiency or of corticotropic failure for patients suffering from septic shock, the current recommendations of the consensus of the Working Group of the American College of Critical Care Medicine are clear.
They notice that the adrenal insufficiency for ICU patients is identified in a better way by an increase in the delta of serum cortisol less than 9 μg / dl, after an ACTH stimulation test at 250 mg or when the random rate of total cortisol is less than 10 mg / dl.37,31
This working group also recommends a more appropriate semantics in order to defining this clinical situation using the following naming: "critical illness-related corticosteroid insufficiency".37,31 Taking into consideration this difficulty to make a diagnostic certainty, the rates of adrenal insufficiency prevalence in ICU are de facto not very accurate and varying. They oscillate from 0 to 77%, depending on the type of the study population, and on the diagnostic criteria.38 Nevertheless, it was proposed to establish 3 prognostic groups during septic shock based on the basal plasma cortisol concentration and adrenal response to a stimulation by ACTH (Figure 1).
Figure 1: Three prognostic groups during septic shock based on the basal plasma cortisol concentration and adrenal response to a stimulation by ACTH
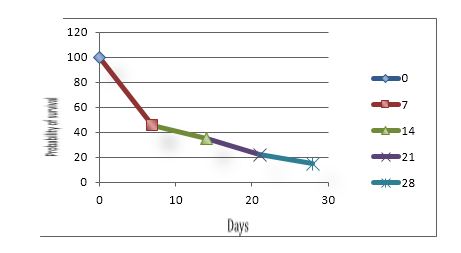
Figure 1A: Basal plasma cortisol level > 34 μg.dl-1, ∆max ≤ 9 μg.dl-1
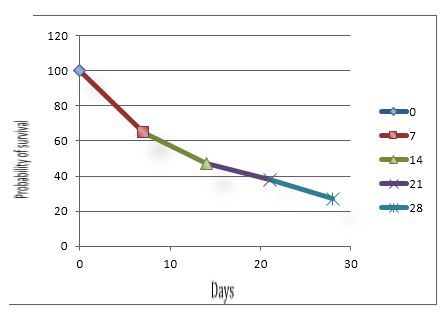
Figure 1B: Basal plasma cortisol level ≤ 34 μg.dl-1, ∆max ≤ 9 μg.dl-1
or basal plasma cortisol level > 34 μg.dl-1, ∆max > 9 μg.dl-1
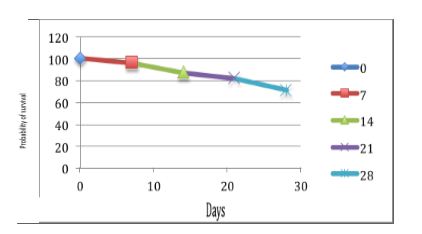
Figure 1C: Basal plasma cortisol level ≤ 34 μg.dl-1, ∆max > 9 μg.dl-1
EFFICACY OF CORTICOSTEROIDS FOR THE TREATMENT OF SEPTIC SHOCK
Until the end of the 1980s, it was admitted to envisage high-dose corticosteroids as an adjuvant therapy of severe sepsis (using either methylprednisolone (30 mg / kg) or dexamethasone (3-6 mg / kg) in divided doses during 1 or 2 days).2,40,41 The rational of this therapeutic strategy was based notably on the prospective and retrospective study results of Schumer et al. These results revealed a survival advantage associated to a high dose corticosteroid treatment.41 However, the relevance of this work would be limited by their retrospective and monocentric character.41
The 50 other therapeutic trials, which were identified over the same period (the 1980s), and which had therapeutic schemes consisting of administering doses ≥ 30 mg.kg-1 of corticosteroid, especially in bolus, for less than 3 days period of time, faced also some limitations due to their low number of persons.
Over the same period, we notice also 9 randomized controlled studies against placebo, led on correct numbers of patients, which documented the efficacy of corticosteroids high dose. These studies resulted in several meta-analyzes.9-11 They have failed, however, to demonstrate any survival benefit for patients suffering from severe sepsis and septic shock.38-40
A sub-population of the selected patients in these clinical trials had developed complications that would be highly linked to this type of treatment.4,9,45
These thinly encouraging results were behind the decision of abandoning the glucocorticoids till the end of the 90’s. During this period, the concept of relative adrenal insufficiency and the recognition of "vasosensitizing" properties of corticosteroids used in low doses have appeared.
Thus, at the end of the 1990s, the emerging data revealed that the use of corticosteroids (termed supraphysiologic dose, stress-dose, or low-dose) for patients affected by septic shock, would significantly improve homeostasis and survival.46,47,48
In fact, Bollaert et al46 published a study comparing the use of hemisuccinate hydrocortisone at a dose of 300 mg/day during 5 days vs. placebo in42 patients suffering from septic shock and who needed catecholamines for more than 48 hours. The results have highlighted a statistically significant improvement of the reversibility of the shock at day 7 (p = 0.007), allowing a dose reduction of amines from day 1. This successful effect on the duration of the shock was strongly correlated to the probability of 28 days survival. It was also comparable to responder or non-responder patients to synachten test done within the 24 hours preceding the inclusion.
All these observations relevant to potential benefit associated to low-dose hydrocortisone for patients suffering from septic shock observed in a clinical trial – of small number of patients - controlled-placebo, have resumed the interest of evaluating the rational of the low dose of steroid therapy for patients suffering from severe sepsis and septic shock.46,47,48,49
Thus, Annane and colleagues49 performed a low-dose corticosteroid trial. In this study, the authors randomized 300 patients suffering from septic shock in two groups; placebo group vs treated group. The regimen of non-placebo group was of 200 mg/day hydrocortisone IV every six hours associated to 50 μg of fludrocortisones per day over a period of seven days. Patients were included within the first 8 hours of shock and after having benefited from a stimulation of 250 μg of ACTH test to the synacthen. The objective of this stimulation was to evaluate the adrenal dysfunction, represented by an increase ≥ to 9 μg / dl of total cortisol compared to the basic rate. The primary end point of 28 day survival was distributed from randomization in the ACTH non-responders. The authors also evaluated overall mortality, days on vasopressin therapy, and adverse events based on steroid replacement versus placebo. In this clinical trial, 76.5% of the study population met the criteria for the ACTH non-responder (Δmax < 9 μg / dl) or adrenal dysfunction. Low- dose steroid treatment was demonstrated to reduce time to shock reversal and mortality.49 The 28-day mortality rate in the hydrocortisone-treated non-responders was 53% versus 63% in the placebo-treated group. Overall, there was a significant improvement in 28-day all-cause mortality rate with low-dose steroid treatment (hazard ratio, 0.71; 95% CI, 0.53–0.97; P 1⁄4 0.03).49 There were no significant differences in adverse events between the two treatment strategies.
It is worth mentioning that Annane and colleagues found a low-dose corticosteroid use to vary by region, with the regional highest use in Europe (more than 50%) and highest individual country use in Brazil (63%).49 However, it is important to mention that some elements of this study might- in some way- be considered as bias. It is mainly the matter of the change of inclusion criteria occurred during the study.
In addition, the use of etomidate, which aims at facilitating endotracheal intubation, for the majority of patients included, might explain partly the high levels of adrenal dysfunction. However, these high levels could indicate the severe situation of the patients included.
Thus, the reported improvement in survival coupled with the smaller findings from studies led the 2004 Surviving Sepsis Campaign to Support the use of low-dose hydrocortisone in patients with vasopressor-dependent septic shock after adequate fluid resuscitation.50
Moreover, among the studies supporting this therapeutic modality, that of Oppert et al should be mentioned.51 It shows an increase in the resolution of septic shock and a decrease of pro-inflammatory cytokines for steroid treated patients with an early hyperdynamic septic shock.46 The therapeutic regimen documented in the framework of this study consisted of administrating a 50 mg of hydrocotisone a bolus IV injection followed by 0.18 mg/kh/h in long-IV) vs placebo.
The results obtained reveal the lack of difference between the two treatment strategies regarding the increase of secondary infections. The time to cessation of vasopressor support (primary endpoint) was significantly shorter in hydrocortisone-treated patients compared with placebo (53 h vs. 120 h, p ≤ 0.02).
However, in the group of treated patients, there is an increase tendency- compared to the placebo-group patients- regarding the insulin-dependence.51
The use of low-dose hydrocortisone therefore is highly relevant thanks to the unanimous results of these studies, showing, on the one hand, a real benefit regarding the survival and the hemodynamic improvement and, on the other hand, the lack of benefit and a possible aggravation in case of the use of high-dose corticosteroid therapy.7,8,11,52
Since then, according to the PROGRESS registry data, counting 12,570 adult patients with severe sepsis recruited between 2002 and 2005, from 276 study centers distributed over 37 countries, in order to evaluate the efficacy of the use of vasopressors and corticosteroids at low doses, nearly 80% of patients received a vasopressor therapy and 35% received low-dose corticosteroids. The data of this register also revealed that, at the macro scale, the rate of use of corticosteroids is the highest in Europe while as it is the lowest in Asia. At the micro scale, Brazil represents 63%, the highest rate of corticosteroid therapy (low dose in the frame of SEPSIS). Malaysia represents 9%, the lowest level.
It is also revealed6,50 that the use of low-dose corticosteroids appears in 14% of patients with severe sepsis who do not need vasopressors. However, after several years of almost globally accepted use, another study on the efficacy of low-dose corticosteroid therapy in septic shock has resumed the controversy.53
This multicenter, randomized, double-blind study had two arms. In one arm, patients underwent a therapy with placebo. In the second arm, patients were administered 50 mg of hydrocortisone every 6 hours, the equivalent of 200 mg / day for 5 days. The total number of patients included was 499 adults who had a septic shock diagnosis.53 Also, the patients had to have hypotension for at least 1 hour and during the first 72 hours of the shock. The primary endpoint was 28-day mortality rate in ACTH no responders (defined as, 9 μg / dl Increase in cortisol after-standard-dose ACTH). The results showed no difference in 28-day mortality between the two arms -the hydrocortisone and placebo treatment groups- (39% vs. 36%, respectively). The finding can also be applied on non-responders patients to synacthen test (p = 0.69). A shock reversal occurred in 3.3 days in the hydrocortisone treatment arm versus 5.8 days for placebo treatment.53
In addition, the steroid- treated patients had a significantly increased frequency of hyperglycemia, hypernatremia, and superinfections, including new episodes of sepsis.53
However, CORTICUS’s study also generated a series of criticisms, just like the case of the study of Annane et al.49
The amendments and changes in the protocol that occurred during the investigation, as well as the limited number due to recruitment difficulties that implied the stopping of the study after the inclusion of only 499 patients while as the needed calculated number was 800 patients, were mentioned among the criticisms. This low number of included patients would be responsible for a significant decrease in power, and in the value of the findings.
Recruitment problems have obliged the authors to include patients "less severe" than those included in the studies described previously. In fact, the definition of “state of shock” in this study is based on Arterial Blood Pressure (ABP) < 90 mm Hg despite the volume expansion or the use of amines. Therefore, a number of included patients presented a severe sepsis and not a septic shock.
This diagnostic element represents a great difference compared to the study of Annane et al in 2002, where only septic shock patients were included. This would explain in part the differences, on the one hand, of the mortality rates observed between control groups of both studies: 36% in the CORTICUS study vs 63% in the study of Annane et al, on the other hand, the slightest proportion of non-responding patients to the synacthen test (46% in CORTICUS study vs 76% in the study of Annane et al in 2002).
Finally, it is worth mentioning that in CORTICUS study, 24% of patients who benefited from a microbiological documentation did not receive an appropriate antibiotic therapy. Thus, based on these elements, the analysis of the non-efficiency of an adjuvant treatment seems difficult.
The results of prospective, randomized, placebo-controlled, double-blind trials of low-dose corticosteroid treatment of patients with septic shock are listed in Table 1.
Another recurrent criticism is emitted at the spot of the corticosteroids evaluation for patients with severe sepsis and / or septic shock. It tackles the potential impact of the frequent use of etomidate, which facilitates intubation, on adrenal response to ACTH stimulation.54
Cuthbertson et al, documented 96 patients who benefited from etomidate during the 72 hours preceding the inclusion to the CORTICUS trial. They were compared to 403 patients who did not receive etomidate during that period.54
The results obtained are indisputable. The Etomidate-treated patients were significantly more likely to be non-responders to ACTH administration (61 vs 44.6%, P ≤ 0.004) and in a univariate analysis, there was an increased mortality associated with etomidate use (odds ratio, 1.70; 95% CI, 1.07-2.68; P ≤ 0.02). The authors also report that the administration of hydrocortisone did not change mortality rate in the Etomidate- treated patients (45% vs 40%).54
It, therefore, easily appears that the results of Annane and colleagues on the one hand, and the CORTICUS trials on the other hand, are discordant and put in perspective the evidence of the systematic recourse to low dose corticosteroid for patients with severe sepsis and septic shock.49,53
Because of the predominance of adverse events within the group of patients treated with the corticosteroid, a re-evaluation of the Surviving Sepsis Campaign recommendation was imperative.
However, the contribution of meta-analyses having tackled this subject remains limited for reasons of notable differences in terms of methodology and population studies.7,52,55,56
In view of this, 17 clinical trials relevant to 28-day mortality were documented by Annane and colleagues. They objectified a higher mortality rate in control group versus corticosteroid-treated group (38.5 vs. 35.3%, respectively).56 The investigators also collected data regarding the impact of super infection and hyperglycemia. The main reported adverse effect was the elevated blood glucose with 51.6% in the corticosteroid arm vs 46% in the placebo arm.
One benefit from the administration of corticosteroids in septic shock is being able to wean patients off vasoactive therapy earlier.56,57 Decreased exposure to vasoactive therapy is potentially beneficial for organ function and peripheral vascular circulation recovery.
Corroborating the results of studies18,57,58 according to which, one important advantage allocated to the administration of corticosteroids in septic shock would be a statistically significant improvement of the reversibility of shock at day 7 (p = 0.007), allowing then dose reduction of catecholamines from day 1.
The results of the trial VAAST, reported meanwhile that the association of low-dose vasopressin and corticosteroids may be linked to a reduction of organ dysfunction and mortality rates compared to the association of norepinephrine and corticosteroids, suggesting the benefits linked to interaction (corticosteroids, vasopressin).57
Generally, the two regimens of the most common corticosteroid are either hydrocortisone 100 mg IV every 8 hours or 50 mg IV every 6 hours. However, to date, these two strategies have not been the subject of any direct comparison. On the other hand, the results of some studies, conducted although on a modest number of patients, to document the effect of hydrocortisone in continuous IV use, noted beneficial effects on hemodynamic function and vasopressor requirement.47,51
Recent Surviving Sepsis Campaign guidelines recommend hydrocortisone dose ≤ 300 mg / d among patients with vasopressor-dependent septic shock but however do not state a preference of one regimen over another.5 There does not appear to be a difference in efficacy, but may there be differences between the two regimens related to the side effects of immune suppression and hyperglycemia.
Furthermore, it is important to highlight that even though the administration of hydrocortisone may be considered either as an IV bolus or by infusion, the rapid intravenous administration provides high spikes in the serum level, and it does not mimic natural cortisol secretion, and results in more dramatic exchange in blood sugar.38 While the continuous infusion administration mimics a more natural physiologic response; however, there appear to be more rebound effects once the corticosteroids-have-been discontinued.38 In addition, current recommendations suggest a downward dose of corticosteroids since the use of vasopressors is no longer useful. Such is to avoid any rebound effect of lowering blood pressure and variability of blood glucose.6, 34
Regarding the role of fludrocortisones therapy associated with hydrocortisone, it was recently been evaluated in the COIITSS Trial. In this trial of 509 patients, the investigators affected patients randomly to one of four treatment groups (intensive glucose control with insulin infusion plus hydrocortisone, intensive glucose control with insulin infusion plus hydrocortisone plus fludrocortisone, conventional insulin therapy with hydrocortisone, or conventional insulin infusion plus hydrocortisone and fludrocortisone).
The approved therapeutic scheme is: hydrocortisone 50 mg every 6 hours and fludrocortisones 50 mg once daily. The authors report that intensive insulin therapy was associated with more frequent episodes of severe hypoglycemia (glucose, 40 mg / dl) with the mean number of episodes of 0.15 (95% CI, 0.02-0.28; P = 0.003).
The survival rate of patients treated by the fludrocortisones is estimated at 105 out of a total of 245 (42.9%) versus 121 of 264 (45.8%) for non-fludrocortisones-treated patients.
In patient group treated with fludrocortisones, an increase in the rate of infections was noted, with predominance for infections related to the urinary organ.
The results of this study also reveal the absence of additional survival benefit associated with treatment and fludrocortisones, perhaps there even may be an increased risk for increased infection.
Based on these results, the relevance of the current recommendations of the Surviving Sepsis Campaign considering fludrocortisones as an optional addition to the low dose hydrocortisone in patients with vasopressor-dependent septic shock clearly is highlighted.6
To note also, these recommendations suggest that low-dose steroids only be used in fluid-resuscitated vasopressor-dependent patients with septic shock, and there is no need to perform an ACTH stimulation or test -other assessment of adrenal function unless adrenal insufficiency is suspected, and to administer treatment for a 7-day course.
UNDESIRABLE EFFECTS AND THERAPEUTIC IMPACT OF CORTICOSTEROIDS
Multiple adverse reactions potentially related to steroid therapy reported by several studies are: gastroduodenal ulcers, infection, delayed wound healing, diabetes decompensation, neuromyopathy. However, although steroids high-dose remains associated with an increase of nosocomial infections, recent trials involving a low-dose hydrocortisone treatment reveal contradictory data. In fact, whereas the results reported by Annane et al reveal a decrease in infectious risks, CORTICUS study reveals an increased risk 1.27 (95% CI, 0.96-1.68). In this study, a recurrence of the shock and initial infection seemed more common for patients receiving corticosteroids [OR: 1.37 (1.05 to 1.79)].
On the one hand, these results could be explained by the use of corticosteroids at anti-inflammatory doses; on the other hand, we may think they are related to the high prevalence of inadequate empirical antibiotic therapy -rather than the specific effect of glucocorticoids. Moreover, the risk of hyperglycemia was 1.18 (95% CI, 1.07-1.31).53
Although, these data must be confirmed by other studies, it’s important to mention that several studies have shown a tendency to hyperglycemia, more pronounced among patients treated with corticosteroids. It is thus recommended to get adequate glycemic control with insulin therapy, regardless of the chosen administration regimen.
Some authors59 are also in favor of a continuous infusion of corticosteroids instead of sequential administration of successive bolus, to allow improved glycemic control. It may be noted that, a study reported an increased incidence of hypernatremia among patients treated with hydrocortisone, but without real clinical consequence.
In light of this multitude inconsistent data some time with high controversy, it is clear that the use of early high-dose corticosteroids is not only not helpful, but seems to be potentially dangerous for patients with severe sepsis and septic shock.7,8,9,57
Indeed, results from multiple controlled clinical trials clearly showed that low-dose corticosteroid replacement therapy in septic shock is associated with improved blood pressure. It is also mentioned an improvement in terms of shorter duration of vasopressor support in patients with septic shock.7,8,44,46,47,49,51,57
However, on the basis of these data, it is absolutely not possible to mention any advantage for survival among patients with vasopressor-dependent septic shock.
In the same frame, we see that the results of recent studies do not provide a sharp answer to the question of whether there is an improvement in all-cause 28-day mortality rate associated with the use of low-dose hydrocortisone replacement therapy in patients with vasopressor -dependent septic shock.49,53
Moreover, Fludrocortisones does not seem to be a necessary adjuvant therapy. It could be associated with an increased risk of infection.
Thus, according to current recommendations, it is appropriate to reserve the use of corticosteroids in patients with septic shock (not severe sepsis) and whose hemodynamic remains unstable despite adequate fluid resuscitation and insufficiently responsive to administration vasopressor.
No catecholamine threshold is set by the authors to define "inadequate blood pressure response" but experts seem to agree on a dose ≥ 0.5 μg/kg/min adrenaline or noradrenalin. It would not be necessary either to realize an exploration of the HPA axis before considering such a course of action.
Regarding the advocated dose of hydrocortisone, according to the authors, it should be less than 300 mg/day, but they do not reach a decision on the duration of treatment. Literature data recommend treatment of 5 to 7 days if the shock persists.
The schemes proposed by experts are then either 200 mg/d in 4 injections of either a bolus of 100 mg followed by a continuous infusion of 240 mg/day.
It is also recommended to start a gradual decrease in doses at the end of treatment to avoid a rebound of shock: 50 mg / 12 h for 3 days and then 50 mg / 24 h for three days before the full stop.
CONCLUSION
Considering the uncertainty regarding the most suitable strategy and optimal duration of low dose, and the absence of homogeneity of diagnostic criteria and evaluations between the various clinical trials, limiting de facto any attempt to compare, it appears necessary to consider other clinical trial types: prospective, randomized, controlled, and double blind with well-defined upstream inclusion and exclusion criteria.
Only then we may conclude some recommendations regarding the potential benefits, the useful duration, the optimal dose and mode of administration (continuous or intermittent, abrupt cessation or by de-escalation) of corticosteroid therapy of vasopressor-dependent septic shock.
Conflict of interests: None
Authors’ Contribution:
AH: Concept, conduction of the study work, literature search and manuscript editing
ML: Conduction of the study, correction
KL: Literature search and manuscript editing
REFERENCES
1Research Unit EA 2694, Public Health Epidemiology & Quality of Care, Medical University, Lille2, (France) 2Research Unit EA 2694, Public Health Epidemiology & Quality of Care, Biostatistics Laboratory, Faculty of Pharmacy ; University Lille2, (France)
3France Accreditation, University Paris Descartes, (France)
Correspondence: Ahmed Haddadi, Research Unit EA 2694, Public Health Epidemiology & Quality of Care, Medical University, Lille2, (France); Tel: +33 6 98 91 16 69; E-mail: ahmed.haddadi@univ-lille2.fr
SUMMARY
The hypothalamic-pituitary-adrenal axis has a great importance in the process of the body's fight against any infection.
In fact, several studies, including reports of an experts’ conference and those of a consensus conference, reveal a high probability of the alteration of the endocrine response in a context of septic shock.
This alteration might appear as ACTH deficiency (adrenocorticotropic deficiency) which pejorative prognosis is attested even if the pathophysiological mechanisms of this inappropriate endocrine response remain poorly understood.
This inappropriate endocrine response might represent thus a rational which is pertinent enough for the use of corticosteroids in the treatment of the septic shock. This rational is enhanced even more on the one hand by a better understanding of the corticosteroids action mechanisms process. On the other hand, by good proved benefit-risk ratio, of the use of corticosteroids, in a given situation dominated by an uncontrolled systemic inflammatory reaction, such as the septic shock.
According to the results of several studies –animal and clinical – the doses of 200 mg /day of hydrocortisone (or equivalent) might allow to restore in a substantial way the hemodynamic and immunizing homeostasis during the severe sepsis and the septic shock.1
A systematic review of randomized trials shows that a corticosteroid therapy at low-dose (200 to 300mg / day of hydrocortisone or equivalent) administered over a long period (at least 5 days of full dose) would improve the 28-day survival at hospital, it would also increase the probability of the shock resolution, it will decrease the period of stay in the intensive care unit and it wouldn’t cause any serious side effects with the exception of metabolic complications.1
However, to date, despite all these provided elements in current practice, the adoption of corticosteroids treatment in septic shock remains very controversial.
This controversy is widely based on the results of some studies that do not only show the absence of any benefit -linked to the use of corticosteroids - but also the possibility of the prognostic aggravation.
Key words: Endocrine; Corticosteroids; Septic shock; Sepsis; Cortisol; Glucocorticoid; Adrenocorticotropic hormone; Inflammatory cytokines
Citation: Haddadi A, Lemdani M, Laribi K. Efficacy of corticosteroids for the treatment of septic shock. Anaesth Pain & Intensive Care 2016;20(4):488-502
Received: 23 Sep 2016; Reviewed: 23 Nov 2016; Corrected: 7 Dec 2016; Accepted: 12 Dec 2016
INTRODUCTION
The physiological functions of cortisol were objectified in the 1930s, and the use of corticosteroids in the treatment of severe infections was mentioned in 1940.1, 2-5
Since then, corticosteroids have proven efficiency in the treatment of some infections.1, 2-4
This is through benefit contribution in survival terms (bacterial meningitis, tuberculosis meningitis, tuberculosis pericarditis, severe typhoid fever, tetanus, moderate to severe pneumocystis) or by long-term benefits (bacterial arthritis). Or finally, over a symptomatically benefit (herpes zoster, infectious mononucleosis, croup, pharyngitis, phlegmon, cellulitis, chronic otitis media, glandular tuberculosis, pleural tuberculosis).1
However, in the frame of severe sepsis and septic shock, their use remains very controversial.
In fact, recent consensus declarations as well as the results of some meta-analyses have reconsidered the recommendations given by Minneci et al. The said recommendations mentioned that a low dose of steroid treatment should be considered almost systematically for every patient suffering from a vasopressor dependent septic shock.6, 7-11
This controversy in the points of view is mainly due to the absence of current tangible clinical proofs or even the ones in progress and to the insufficient multiple meta-analysis results in the frame of answering the following questions: Is a corticosteroid therapy really beneficial for patients suffering from severe sepsis and septic shock, What is the best dose recommended, Should the treatment be intermittent or continuous, Should its interruption be brutal or based on a decreasing process, Should the treatment duration be fixed or should it depend on the clinical response.
Thus, the aim of this study tackles, after the explanation of the concept of the relative adrenal insufficiency associated with the septic shock, a systematic analysis of the randomized trials over the substitutive opotherapy in septic shock and severe sepsis.
CORTISOL PHYSIOLOGY
Cortisol is the main glucocorticoid. It is a steroid hormone composed of 19 carbon atoms synthesized from cholesterol by the cytochrome enzyme P450. It is secreted by the zona fasciculata, which is the deepest adrenal corticosteroid area.
It circulates in the plasma as an active free form (5-10% of total cortisol), or as an inactive form reversibly linked mainly to the following two binding proteins: cortisol binding protein (CBG), and albumin.13
Its penetration into cells is passive; it binds with a specific cytosolic soluble receptor, glucocorticoid receptor (GR) Type II.
This connection, characterized by the cortisol and its receptor, activates the complex cortisol-GR by a process involving dissociation of the proteins known as chaperones, especially those of heat shock 90 and 70 and FK-506 binding proteins).14
Thus, the complex cortisol-GR can migrate actively (ATP dependent) in the nucleus where it plays the transcription factor role.
It interacts with several specific DNA sites located in the region promoting target genes, activating several genes transcription.
Nevertheless, the complex cortisol-GR can also interact at the cell membrane level or with different cytosolic proteins especially with other transcription factors, influencing the activity of the latter on their own target genes.
The cortisol is metabolized in the liver and the kidney.15 It is worth mentioning that it is transformed into inactive metabolite, cortisone, by the enzyme 11-β-HSD2. However, due to 11-β-HSD1, several tissues, including the liver, the adipose tissue, and the bone tissue have the ability to convert the inactive cortisone to active cortisol.16
REGULATING ROLE OF THE HYPOTHALAMUS-PITUITARY AXIS
The adrenocorticotropic hormone (ACTH), produced by the anterior pituitary gland stimulates largely the cortisol synthesis and secretion.17
However, the secretory regulation of ACTH depends on several factors, including the corticotropin-releasing hormone (CRH), which is secreted by the hypothalamus and liberated in the hypothalamus-pituitary axis.
In this intricate scheme and interdependence, we will notice that arginine vasopressin (AVP) stimulates weakly ACTH secretion, but it increases the CRH action. Besides, catecholamines, angiotensin II, serotonin and vasoactive intestinal peptide (VIP) are stimulating elements of the ACTH secretion.
As for ACTH secretion, some inflammatory cytokines such as interleukin IL-1, IL-2, IL-6, tumor necrosis factor (TNF) are naturally stimulating. However, other cytokines such as the transforming growth factor are inhibitors.
As regards the CRH, its production is stimulated by adrenergic agonists (noradrenalin), serotonin and inhibited by the substance P, the opiates and the γ-aminobutyric acid.
Inflammatory cytokines (IL-1, IL-2, IL-6, and TNF) influence the CRH production.
Finally, on the one hand, it is worth mentioning that there is a negative feedback of glucocorticoids on the corticotropic axis which is revealed as an inhibition of the production of ACTH, CRH and AVP.
On the other hand, the secretion of the corticotropic axis hormones is pulsating during the circadian cycle reaching its maximum in the morning between 6 am and 8 am, then it decreases rapidly until noon, afterwards it registers a slower decrease until midnight.18
GLUCOCORTICOIDS PHYSIOLOGICAL EFFECTS ON THE MAIN ORGANS AND FUNCTIONS
Metabolic effects
The glucocorticoids are highly involved in glucose metabolism. In the liver, they stimulate the gluconeogenesis and the glycogenolysis. By increasing peripheral resistance to insulin, they inhibit the glucose cell extraction leading thus to the increase of the blood glucose.19 Beyond stimulating the glucagon and adrenaline secretion, they also affect lipid metabolism, they stimulate the lipolysis and decrease the glucose consumption by adipocytes.
It is worth mentioning, however, that cortisol inhibits the protein synthesis, it activates the muscle protein breakdown liberating thus amino acids serving as substrate for gluconeogenesis. At the bone level, the glucocorticoid action on calcium metabolism is acknowledged.
Furthermore, cortisol activates the osteoclasts, inhibits the osteoblasts, inhibits the intestinal absorption of calcium, and increases the urinary secretion of calcium by decreasing renal re-absorption.
Immunological and anti-inflammatory effects
The immunomodulator and anti-inflammatory effects of cortisol have been widely documented. However, to date, they are still weakly clarified.20
Nevertheless, it is proved that cortisol acts almost over the entire cellular panoply of immunity (poly-nuclear neutrophils, lymphocytes, monocytes, macrophages, eosinophils, basophils), by affecting important cellular functions, such as migration and chemotaxis, apoptosis, phagocytosis, anti-oxidative metabolism, adhesion and communication (cytokine production).
It promotes the lymphocytes migration starting the circulation towards lymphoid organs. It is also behind the migration of neutrophils and macrophages inhibition towards inflammation sites, which leads to the decrease of local inflammation.
Even though glucocorticoids have a stimulating action of eosinophil apoptosis, they are originally protectors of monocyte apoptosis.
In humoral immunity, cortisol has a definite role as a modulator since it inhibits the IL-12 production by the macrophages and monocytes, which affects de facto the lymphocyte differentiation by acting on the balance of Th1-Th2 in favor of Th2 cells. The IL-4 secretion (which is normally inhibited by IL-12) is then increased.
The promotion of Th2 activity and humoral immunity is joined to the suppression of cellular immunity, noting that Th1 and Th2 activities are mutually inhibitory. However, all these observations objectified in vitro must be considered with some reservation waiting to be confirmed in vivo.
At the cellular level, the inflammatory response is modulated by cortisol.
In fact, even though glucocorticoids slow down the synthesis or the action of the majority of pro-inflammatory cytokines (IL-1, IL-2, IL-3, IL-6, IFN, TNF), of chemokines, eicosanoids, bradykinin, and MIF, they stimulate the production of many anti-inflammatory factors such as the receptor agonist to IL-1, soluble TNF receptor, IL-10 and the transforming growth factor β.
This anti-inflammatory action is consolidated by inhibiting cyclooxygenase production and NO synthase induced form, which are inflammation key enzymes.
Finally, it has been demonstrated that cortisol has an action on over nearly 2,000 genes involved in immune response.21
Cardiovascular effects
Cortisol action on the kidney and vascular endothelium has an essential role in cardiovascular homeostasis.22 In fact, its action takes part in preserving the vascular tension, the vascular permeability and the total distribution of H2O volume in the vascular compartment. Its action mechanisms on the cardiovascular system remain, to date, poorly understood, but nonetheless they seem to be independent of mineral corticoids effects and of sympathetic system.23,24
In the smooth muscle, cortisol increases sensitivity to vasoconstrictor agents such as catecholamines and angiotensin II, especially by increasing the transcription and expression of receptors to these hormones.25
The cortisol effects on nitric oxide are complex; it increases the synthesis of the endothelial form of the nitric oxide synthase, maintaining thus the tissue perfusion.26 The glucocorticoids effects on the vasomotor tone are probably premature (within minutes) and they have a non-genomic mechanism.
Glucocorticoids molecular action mechanisms
The molecular action process of glucocorticoids is complex and remains, to date, not completely clarified.17,20,21 However, separate effects should be mentioned: non-genomic effects and genomic effects. Non-genomic effects are premature, occurring a few minutes after corticosteroids administration. They are either induced directly by interaction with membrane sites or linked to the release of chaperone proteins during the formation of glucocorticoids complex- GR. The first genomic effects are rather of late appearance. They require several hours of exposure to a glucocorticoid. In this case, the involved phenomenon is that of transrepression by sequestering in the cytoplasm the nuclear transcription factors such as NF- kB and AP-1, avoiding the transcription of genes for almost all the pro-inflammatory mediators.27,28
In addition, the NF-kB is normally kept as an inactive form in the cytoplasm by interaction with inhibitory proteins (IkB). However, the NF-kB / IkB complex is activated by phosphorylation and IkB protein degradation.
When the NF-KB is released, it migrates into the nucleus and binds to the promoter regions of target genes in order to initiate the transcription of multiple cytokines (tumor necrosis factor α, IL-1, IL-6), adhesion cells (intracellular adhesion molecul-1, E-selectin) and other inflammatory mediators.
The role of glucocorticoids is also the stimulation of IkB transcription.
Thus, the prominent role of the transactivation mechanisms in modulating the immune response by corticosteroids was also recently demonstrated.21
It is worth mentioning that the genomic transactivation represents a notably delayed response, occurring few days after the glucocorticoids administration (but some genes are also trans-activated in the first hours such as annexin-1, 2 adrenergic receptors and protein phosphatase 2).
The glucocorticoid-GR complex acts then as a transcription factor: it migrates into the nucleus and binds to target genes activating their transcription. Immune cells, such as monocytes, are then reprogrammed with cellular function modulation such as apoptosis, adhesion, cell motility, chemotaxis, phagocytosis, anti-oxidative reactions.
CORTICOSTEROID INSUFFICIENCY IN SEPTIC SHOCK
It is confirmed that in order to respond normally to a stress resulting from any type of serious acute pathology, the plasma cortisol has to be increased. It aims at maintaining the homeostatic balance.17,29
The processes brought into this hypercortisolemia are:
1-The increase of cortisol synthesis;
2- The increase of the conversion of cortisone into cortisol;
3- The decrease of the cortisol clearance;
4- The increase of free cortisol.
During stress, the production of cortisol is incidentally subordinated to the production of CRH and ACTH. Pro-inflammatory cytokines and the sympathetic nervous system become thus the important modulators of the corticotropic axis.29
Then, cortisol is synthesized in a continuous way instead of a pulsatile way, registering thus a loss of circadian rhythm and a decrease of the negative feedback capacity of cortisol on the hypothalamic-pituitary hormones.
However, the conversion of the cortisone into cortisol (catalyzed by 11- β -HSD1) is increased by the stromal cells such as fibroblasts.16
In case a renal and hepatic blood flow reduction is registered, then the cortisol clearance by the liver and the kidney might be pejoratively affected.15 This situation may also lead to increase the free cortisol concentrations and to rapidly decrease the CBG (Corticosteroid-binding globulin) and albumin concentrations.
In addition, it is important to note that in a stress context, the total rate of cortisol measured in the serum does not necessarily reflect that of the active free cortisol. The latter is probably underestimated.30
Animal and clinical studies suggest that sepsis may alter cortisol metabolism.31
On the contrary, pejorative and harmful implication of the corticosteroid insufficiency in sepsis towards the septic shock and death is quite probable.32,33
We have to fear the appearance of suprarenal insufficiency during the sepsis when the patients suffer from a hypothalamic-pituitary axis (e.g. Addison's disease) and who have been undergoing a corticosteroid therapy for more than seven days, regardless of the galenic form, as well as the patients diagnosed with sepsis and who have been exposed to treatments blocking the cortisol adrenal synthesis.34
A weak inappropriate production of cortisol synthesis (primary or secondary adrenal insufficiency) as well as an increased tissue resistance to glucocorticoids is probable.35
However, we have several processes of corticosteroid insufficiency in severe sepsis and septic shock which remain unclear to date.
The etiological cause of adrenal insufficiency (decrease of cortisol synthesis) in septic shock might be of pituitary origin (or rarely hypothalamic) when it is secondary. It is then characterized by an increase of ACTH production (or CRH production).
It might be of adrenal origin when it is primary. In this case, this type of insufficiency might follow the necrosis or hypothalamus hemorrhage or pituitary gland hemorrhage (if there is prolonged hypotension or severe coagulation disorders). It might follow as well a chronic or a latent secondary adrenal insufficiency degradation (hypothalamic or pituitary tumor, chronic congenital inflammation), or iatrogenic factors (corticosteroids therapy, opiates...).
The hemorrhage or the adrenal bilateral necrosis, the viral inflammation (HIV) or fungal inflammation, and the enzymatic cascade change that converts cholesterol to cortisol (e.g. etomidate) are also the pertinent causes of primary adrenal insufficiency.
However, there is also probably a tissue resistance to glucocorticoids, following a quantitative or qualitative alteration of the glucocorticoids receptors (this argument lacks evidence in vivo), through an increased activity of 11-β-hydroxysteroid dehydrogenase, an abnormal conversion of cortisol into inactive cortisone, an alteration of the cortisol transportation phenomena both at the systemic level (decrease in circulating CBG and albumin), the tissue level (abnormal cleavage of CBG-cortisol complex caused by elastase’s defect) and the cellular level (cortisol rejection outside the cell by proteins of "cleansing").
DIAGNOSIS
It is still hard to establish the diagnosis of the confirmed adrenal insufficiency during the sepsis for ICU patients. This is due to the non-specificity of clinical signs (fever, nausea, vomiting, abdominal pain, consciousness disorders, and hypotension) and biological signs (hypoglycemia, hyponatremia, hyper eosinophilia).34
As for the static hormones dosages do not allow to confirm the diagnosis with the exception of rare cases where the cortisol concentration is < 3 μg/dL.
It is important to highlight the limits of the total plasma cortisol concentration because it does not always reflect the free cortisol (especially in the case of hypoalbuminemia and during severe sepsis).30 Even when high levels of cortisol during sepsis are objectified it cannot be by itself a decisive diagnosis element neither a sign of a good adrenal function. It should suggest possible consequence of a defect elimination or peripheral resistance,15 hence the interest of a dynamic test to evaluate adrenal function.34
The traditional evaluation method used in intensive care units is the ACTH stimulation test, through the administration of 1 μg (low-dose short test) or 250 μg (conventional-dose) of cosyntropin.
However, the sensitivity and the specificity of this diagnostic test are debatable.34,36 The sensitivity of the synacthen test during the septic shock would be 68% with a specificity of 65%.5 Several experimental studies.29] have also demonstrated the reproducibility absence of the dynamic test in stress situations for the same person and the great variability in dosage techniques. Some authors have set then a total threshold value of cortisol in the case of a physiological stimulation (e.g. state of shock) or after stimulation by ACTH (low or high dose).36
The studies of Salgado and all have evaluated several diagnosis approaches of the alteration of adrenal function where ICU patients suffer from a septic shock. These studies revealed that an increase of cortisol total levels ≤ 9 μg / dL compared to the value at start, 60 min after a standard stimulation test at a dose of ACTH 250 μg might be the best prognostic criterion of the corticotropic insufficiency.36 Based on this diagnosis criterion, among the 102 patients suffering from septic shock and tested in the study of Salgado et al, 22.5% presented an adrenal dysfunction.
Another clinical trial that compares the ACTH stimulation at low dose vs. a standard dose - in the frame of the evaluation of the adrenal function- reveals that the absence of response to low-dose stimulation (1 μg of ACTH) might be correlated to a poor survival rate. For some of these patients with the absence of response, the standard dose test would not be of best interest.37
It is, however, important to mention that the ACTH stimulation tests only the response of the adrenal glands and does not evaluate the HPA axis.27
However, despite the persistent debate on diagnostic modalities of insufficiency or of corticotropic failure for patients suffering from septic shock, the current recommendations of the consensus of the Working Group of the American College of Critical Care Medicine are clear.
They notice that the adrenal insufficiency for ICU patients is identified in a better way by an increase in the delta of serum cortisol less than 9 μg / dl, after an ACTH stimulation test at 250 mg or when the random rate of total cortisol is less than 10 mg / dl.37,31
This working group also recommends a more appropriate semantics in order to defining this clinical situation using the following naming: "critical illness-related corticosteroid insufficiency".37,31 Taking into consideration this difficulty to make a diagnostic certainty, the rates of adrenal insufficiency prevalence in ICU are de facto not very accurate and varying. They oscillate from 0 to 77%, depending on the type of the study population, and on the diagnostic criteria.38 Nevertheless, it was proposed to establish 3 prognostic groups during septic shock based on the basal plasma cortisol concentration and adrenal response to a stimulation by ACTH (Figure 1).
Figure 1: Three prognostic groups during septic shock based on the basal plasma cortisol concentration and adrenal response to a stimulation by ACTH

Figure 1A: Basal plasma cortisol level > 34 μg.dl-1, ∆max ≤ 9 μg.dl-1

Figure 1B: Basal plasma cortisol level ≤ 34 μg.dl-1, ∆max ≤ 9 μg.dl-1
or basal plasma cortisol level > 34 μg.dl-1, ∆max > 9 μg.dl-1

Figure 1C: Basal plasma cortisol level ≤ 34 μg.dl-1, ∆max > 9 μg.dl-1
EFFICACY OF CORTICOSTEROIDS FOR THE TREATMENT OF SEPTIC SHOCK
Until the end of the 1980s, it was admitted to envisage high-dose corticosteroids as an adjuvant therapy of severe sepsis (using either methylprednisolone (30 mg / kg) or dexamethasone (3-6 mg / kg) in divided doses during 1 or 2 days).2,40,41 The rational of this therapeutic strategy was based notably on the prospective and retrospective study results of Schumer et al. These results revealed a survival advantage associated to a high dose corticosteroid treatment.41 However, the relevance of this work would be limited by their retrospective and monocentric character.41
The 50 other therapeutic trials, which were identified over the same period (the 1980s), and which had therapeutic schemes consisting of administering doses ≥ 30 mg.kg-1 of corticosteroid, especially in bolus, for less than 3 days period of time, faced also some limitations due to their low number of persons.
Over the same period, we notice also 9 randomized controlled studies against placebo, led on correct numbers of patients, which documented the efficacy of corticosteroids high dose. These studies resulted in several meta-analyzes.9-11 They have failed, however, to demonstrate any survival benefit for patients suffering from severe sepsis and septic shock.38-40
A sub-population of the selected patients in these clinical trials had developed complications that would be highly linked to this type of treatment.4,9,45
These thinly encouraging results were behind the decision of abandoning the glucocorticoids till the end of the 90’s. During this period, the concept of relative adrenal insufficiency and the recognition of "vasosensitizing" properties of corticosteroids used in low doses have appeared.
Thus, at the end of the 1990s, the emerging data revealed that the use of corticosteroids (termed supraphysiologic dose, stress-dose, or low-dose) for patients affected by septic shock, would significantly improve homeostasis and survival.46,47,48
In fact, Bollaert et al46 published a study comparing the use of hemisuccinate hydrocortisone at a dose of 300 mg/day during 5 days vs. placebo in42 patients suffering from septic shock and who needed catecholamines for more than 48 hours. The results have highlighted a statistically significant improvement of the reversibility of the shock at day 7 (p = 0.007), allowing a dose reduction of amines from day 1. This successful effect on the duration of the shock was strongly correlated to the probability of 28 days survival. It was also comparable to responder or non-responder patients to synachten test done within the 24 hours preceding the inclusion.
All these observations relevant to potential benefit associated to low-dose hydrocortisone for patients suffering from septic shock observed in a clinical trial – of small number of patients - controlled-placebo, have resumed the interest of evaluating the rational of the low dose of steroid therapy for patients suffering from severe sepsis and septic shock.46,47,48,49
Thus, Annane and colleagues49 performed a low-dose corticosteroid trial. In this study, the authors randomized 300 patients suffering from septic shock in two groups; placebo group vs treated group. The regimen of non-placebo group was of 200 mg/day hydrocortisone IV every six hours associated to 50 μg of fludrocortisones per day over a period of seven days. Patients were included within the first 8 hours of shock and after having benefited from a stimulation of 250 μg of ACTH test to the synacthen. The objective of this stimulation was to evaluate the adrenal dysfunction, represented by an increase ≥ to 9 μg / dl of total cortisol compared to the basic rate. The primary end point of 28 day survival was distributed from randomization in the ACTH non-responders. The authors also evaluated overall mortality, days on vasopressin therapy, and adverse events based on steroid replacement versus placebo. In this clinical trial, 76.5% of the study population met the criteria for the ACTH non-responder (Δmax < 9 μg / dl) or adrenal dysfunction. Low- dose steroid treatment was demonstrated to reduce time to shock reversal and mortality.49 The 28-day mortality rate in the hydrocortisone-treated non-responders was 53% versus 63% in the placebo-treated group. Overall, there was a significant improvement in 28-day all-cause mortality rate with low-dose steroid treatment (hazard ratio, 0.71; 95% CI, 0.53–0.97; P 1⁄4 0.03).49 There were no significant differences in adverse events between the two treatment strategies.
It is worth mentioning that Annane and colleagues found a low-dose corticosteroid use to vary by region, with the regional highest use in Europe (more than 50%) and highest individual country use in Brazil (63%).49 However, it is important to mention that some elements of this study might- in some way- be considered as bias. It is mainly the matter of the change of inclusion criteria occurred during the study.
In addition, the use of etomidate, which aims at facilitating endotracheal intubation, for the majority of patients included, might explain partly the high levels of adrenal dysfunction. However, these high levels could indicate the severe situation of the patients included.
Thus, the reported improvement in survival coupled with the smaller findings from studies led the 2004 Surviving Sepsis Campaign to Support the use of low-dose hydrocortisone in patients with vasopressor-dependent septic shock after adequate fluid resuscitation.50
Moreover, among the studies supporting this therapeutic modality, that of Oppert et al should be mentioned.51 It shows an increase in the resolution of septic shock and a decrease of pro-inflammatory cytokines for steroid treated patients with an early hyperdynamic septic shock.46 The therapeutic regimen documented in the framework of this study consisted of administrating a 50 mg of hydrocotisone a bolus IV injection followed by 0.18 mg/kh/h in long-IV) vs placebo.
The results obtained reveal the lack of difference between the two treatment strategies regarding the increase of secondary infections. The time to cessation of vasopressor support (primary endpoint) was significantly shorter in hydrocortisone-treated patients compared with placebo (53 h vs. 120 h, p ≤ 0.02).
However, in the group of treated patients, there is an increase tendency- compared to the placebo-group patients- regarding the insulin-dependence.51
The use of low-dose hydrocortisone therefore is highly relevant thanks to the unanimous results of these studies, showing, on the one hand, a real benefit regarding the survival and the hemodynamic improvement and, on the other hand, the lack of benefit and a possible aggravation in case of the use of high-dose corticosteroid therapy.7,8,11,52
Since then, according to the PROGRESS registry data, counting 12,570 adult patients with severe sepsis recruited between 2002 and 2005, from 276 study centers distributed over 37 countries, in order to evaluate the efficacy of the use of vasopressors and corticosteroids at low doses, nearly 80% of patients received a vasopressor therapy and 35% received low-dose corticosteroids. The data of this register also revealed that, at the macro scale, the rate of use of corticosteroids is the highest in Europe while as it is the lowest in Asia. At the micro scale, Brazil represents 63%, the highest rate of corticosteroid therapy (low dose in the frame of SEPSIS). Malaysia represents 9%, the lowest level.
It is also revealed6,50 that the use of low-dose corticosteroids appears in 14% of patients with severe sepsis who do not need vasopressors. However, after several years of almost globally accepted use, another study on the efficacy of low-dose corticosteroid therapy in septic shock has resumed the controversy.53
This multicenter, randomized, double-blind study had two arms. In one arm, patients underwent a therapy with placebo. In the second arm, patients were administered 50 mg of hydrocortisone every 6 hours, the equivalent of 200 mg / day for 5 days. The total number of patients included was 499 adults who had a septic shock diagnosis.53 Also, the patients had to have hypotension for at least 1 hour and during the first 72 hours of the shock. The primary endpoint was 28-day mortality rate in ACTH no responders (defined as, 9 μg / dl Increase in cortisol after-standard-dose ACTH). The results showed no difference in 28-day mortality between the two arms -the hydrocortisone and placebo treatment groups- (39% vs. 36%, respectively). The finding can also be applied on non-responders patients to synacthen test (p = 0.69). A shock reversal occurred in 3.3 days in the hydrocortisone treatment arm versus 5.8 days for placebo treatment.53
In addition, the steroid- treated patients had a significantly increased frequency of hyperglycemia, hypernatremia, and superinfections, including new episodes of sepsis.53
However, CORTICUS’s study also generated a series of criticisms, just like the case of the study of Annane et al.49
The amendments and changes in the protocol that occurred during the investigation, as well as the limited number due to recruitment difficulties that implied the stopping of the study after the inclusion of only 499 patients while as the needed calculated number was 800 patients, were mentioned among the criticisms. This low number of included patients would be responsible for a significant decrease in power, and in the value of the findings.
Recruitment problems have obliged the authors to include patients "less severe" than those included in the studies described previously. In fact, the definition of “state of shock” in this study is based on Arterial Blood Pressure (ABP) < 90 mm Hg despite the volume expansion or the use of amines. Therefore, a number of included patients presented a severe sepsis and not a septic shock.
This diagnostic element represents a great difference compared to the study of Annane et al in 2002, where only septic shock patients were included. This would explain in part the differences, on the one hand, of the mortality rates observed between control groups of both studies: 36% in the CORTICUS study vs 63% in the study of Annane et al, on the other hand, the slightest proportion of non-responding patients to the synacthen test (46% in CORTICUS study vs 76% in the study of Annane et al in 2002).
Finally, it is worth mentioning that in CORTICUS study, 24% of patients who benefited from a microbiological documentation did not receive an appropriate antibiotic therapy. Thus, based on these elements, the analysis of the non-efficiency of an adjuvant treatment seems difficult.
The results of prospective, randomized, placebo-controlled, double-blind trials of low-dose corticosteroid treatment of patients with septic shock are listed in Table 1.
| Study | Study Population
N : effectif |
Steroid Treatment | Primary Outcome | 28-Day Placebo Mortality (%) | 28-Day Treatment Mortality (%) | Conclusion |
| Schumer (41) |
Septic shock 172 |
(1) Dexamethasone (3 mg/kg as a single intravenous bolus); (2) methylprednisolone (30 mg/kg as a single intravenous bolus); (3) placebo Treatment may have been repeated once after 4 h and had to be initiated at the time of diagnosis. |
28-d Mortality | 10.4 | 38.4 | Hydrocortisone treatment significantly improved survival |
| Bone (42) |
Septic shock severe 382 (sepsis n=234; septic shock, n=148) |
(1)Methylprednisolone (30-mg/kg 20-min intravenous infusion every 6 h for 24 h); (2) placebo Treatment had to be initiated by 2 h after time entry criteria were met. |
14-d development of shock for severe sepsis |
29 | 59 | No significant differences were found in the prevention of shock, the reversal of shock, or overall mortality |
| Bollaert (46) | Vasopressor-dependent septic shock on ventilator for >48 h 41 |
Hydrocortisone 100 mg q8h for 5 d, then wean over 6 d | Shock reversal | 63 | 32 | Hydrocortisone treatment significantly improved hemodynamic abnormalities of septic shock |
| Briegel (47) | Vasopressor-dependent septic shock on ventilator 40 |
Hydrocortisone 100 mg load, then 0.18 mg/kg/h continuous infusion until reversal of shock, then wean over 6 d | Shock reversal | 30 | 20 | Hydrocortisone treatment significantly decreased time to cessation of vasopressor treatment |
| Yildiz (48) | Patients with sepsis (ACCP-SCCM criteria) (52) 40 |
Prednisolone 5 mg at 06:00 and 2.5 mg at 18:00 for 10 d | 28-d All-cause mortality | 60 | 40 | Trend toward decreased mortality with physiological-dose steroid treatment |
| Annane (49) | Vasopressor-dependent septic shock 300 |
Hydrocortisone 50 mg q6h for 7 d and fludrocortisone 50 μg daily for 7 d | 28-d Survival distribution from randomization in nonresponders | 63 | 53 | Hydrocortisone treatment significantly improved survival and shock reversal in nonresponders to ACTH stimulation test |
| Oppert (51) |
Vasopressor-dependent septic shock 40 |
Hydrocortisone 50 mg bolus, then 0.18 mg/kg/h until vasopressor discontinued, then wean to 0.06 mg/kg/h for 24 h, then reduced by 0.02 mg/kg/h/d until off | Time to vasopressor discontinuation | 48 | 39 | Hydrocortisone treatment significantly improved shock reversal and decreased level of proinflammatory cytokines |
| Sprung (53) | Septic shock 499 |
Hydrocortisone 50 mg q6h for 5 d then 50 mg q12h for 3 d, then 50 mg q24h for 3 d | 28-d Mortality rate in nonresponders to ACTH stimulation test | 36.1 in Nonresponders, 31.5 overall | 39.2 in Nonresponders, 34.3 overall | Hydrocortisone treatment did not significantly improve 28-d survival or shock reversal in septic shock nonresponders to ACTH stimulation test |
| VASSCSG1987 (60) |
Septic shock Severe 223 (sepsis n=123; septic shock, n=100) |
(1) Methylprednisolone (30 mg/kg as a single intravenous 10- to 15-min infusion followed by a constant infusion of 5 mg/kg/h for 9 h); (2) placebo Treatment had to be initiated within 2h |
14-d mortality | 22 | 21 | The principal end point was similar in the two groups |
| Cicarelli et al (61) |
Septic shock Severe 29 |
(1) Dexamethasone (0.2 mg/kg intravenously, 3 doses at intervals of 36 h); (2) placebo | Duration of vasopressor Use 7-day mortality |
67 | 21 | Early treatment with dexamethasone reduced the seven-day mortality among septic shock patients and showed a trend towards reduction of 28-day mortality. |
| Chawla et al7 1999 (62) |
Vasopressor- dependent septic shock 44 |
(1) Hydrocortisone (100-mg intravenous bolus every 8 h for 3 d then weaning over 4 d); (2) placebo Treatment had to be initiated 72 h after shock onset.. |
Shock reversal | 36 | 70 | Hydrocortisone improves hemodynamics and reverses shock in patients with refractory septic shock |
Another recurrent criticism is emitted at the spot of the corticosteroids evaluation for patients with severe sepsis and / or septic shock. It tackles the potential impact of the frequent use of etomidate, which facilitates intubation, on adrenal response to ACTH stimulation.54
Cuthbertson et al, documented 96 patients who benefited from etomidate during the 72 hours preceding the inclusion to the CORTICUS trial. They were compared to 403 patients who did not receive etomidate during that period.54
The results obtained are indisputable. The Etomidate-treated patients were significantly more likely to be non-responders to ACTH administration (61 vs 44.6%, P ≤ 0.004) and in a univariate analysis, there was an increased mortality associated with etomidate use (odds ratio, 1.70; 95% CI, 1.07-2.68; P ≤ 0.02). The authors also report that the administration of hydrocortisone did not change mortality rate in the Etomidate- treated patients (45% vs 40%).54
It, therefore, easily appears that the results of Annane and colleagues on the one hand, and the CORTICUS trials on the other hand, are discordant and put in perspective the evidence of the systematic recourse to low dose corticosteroid for patients with severe sepsis and septic shock.49,53
Because of the predominance of adverse events within the group of patients treated with the corticosteroid, a re-evaluation of the Surviving Sepsis Campaign recommendation was imperative.
However, the contribution of meta-analyses having tackled this subject remains limited for reasons of notable differences in terms of methodology and population studies.7,52,55,56
In view of this, 17 clinical trials relevant to 28-day mortality were documented by Annane and colleagues. They objectified a higher mortality rate in control group versus corticosteroid-treated group (38.5 vs. 35.3%, respectively).56 The investigators also collected data regarding the impact of super infection and hyperglycemia. The main reported adverse effect was the elevated blood glucose with 51.6% in the corticosteroid arm vs 46% in the placebo arm.
One benefit from the administration of corticosteroids in septic shock is being able to wean patients off vasoactive therapy earlier.56,57 Decreased exposure to vasoactive therapy is potentially beneficial for organ function and peripheral vascular circulation recovery.
Corroborating the results of studies18,57,58 according to which, one important advantage allocated to the administration of corticosteroids in septic shock would be a statistically significant improvement of the reversibility of shock at day 7 (p = 0.007), allowing then dose reduction of catecholamines from day 1.
The results of the trial VAAST, reported meanwhile that the association of low-dose vasopressin and corticosteroids may be linked to a reduction of organ dysfunction and mortality rates compared to the association of norepinephrine and corticosteroids, suggesting the benefits linked to interaction (corticosteroids, vasopressin).57
Generally, the two regimens of the most common corticosteroid are either hydrocortisone 100 mg IV every 8 hours or 50 mg IV every 6 hours. However, to date, these two strategies have not been the subject of any direct comparison. On the other hand, the results of some studies, conducted although on a modest number of patients, to document the effect of hydrocortisone in continuous IV use, noted beneficial effects on hemodynamic function and vasopressor requirement.47,51
Recent Surviving Sepsis Campaign guidelines recommend hydrocortisone dose ≤ 300 mg / d among patients with vasopressor-dependent septic shock but however do not state a preference of one regimen over another.5 There does not appear to be a difference in efficacy, but may there be differences between the two regimens related to the side effects of immune suppression and hyperglycemia.
Furthermore, it is important to highlight that even though the administration of hydrocortisone may be considered either as an IV bolus or by infusion, the rapid intravenous administration provides high spikes in the serum level, and it does not mimic natural cortisol secretion, and results in more dramatic exchange in blood sugar.38 While the continuous infusion administration mimics a more natural physiologic response; however, there appear to be more rebound effects once the corticosteroids-have-been discontinued.38 In addition, current recommendations suggest a downward dose of corticosteroids since the use of vasopressors is no longer useful. Such is to avoid any rebound effect of lowering blood pressure and variability of blood glucose.6, 34
Regarding the role of fludrocortisones therapy associated with hydrocortisone, it was recently been evaluated in the COIITSS Trial. In this trial of 509 patients, the investigators affected patients randomly to one of four treatment groups (intensive glucose control with insulin infusion plus hydrocortisone, intensive glucose control with insulin infusion plus hydrocortisone plus fludrocortisone, conventional insulin therapy with hydrocortisone, or conventional insulin infusion plus hydrocortisone and fludrocortisone).
The approved therapeutic scheme is: hydrocortisone 50 mg every 6 hours and fludrocortisones 50 mg once daily. The authors report that intensive insulin therapy was associated with more frequent episodes of severe hypoglycemia (glucose, 40 mg / dl) with the mean number of episodes of 0.15 (95% CI, 0.02-0.28; P = 0.003).
The survival rate of patients treated by the fludrocortisones is estimated at 105 out of a total of 245 (42.9%) versus 121 of 264 (45.8%) for non-fludrocortisones-treated patients.
In patient group treated with fludrocortisones, an increase in the rate of infections was noted, with predominance for infections related to the urinary organ.
The results of this study also reveal the absence of additional survival benefit associated with treatment and fludrocortisones, perhaps there even may be an increased risk for increased infection.
Based on these results, the relevance of the current recommendations of the Surviving Sepsis Campaign considering fludrocortisones as an optional addition to the low dose hydrocortisone in patients with vasopressor-dependent septic shock clearly is highlighted.6
To note also, these recommendations suggest that low-dose steroids only be used in fluid-resuscitated vasopressor-dependent patients with septic shock, and there is no need to perform an ACTH stimulation or test -other assessment of adrenal function unless adrenal insufficiency is suspected, and to administer treatment for a 7-day course.
UNDESIRABLE EFFECTS AND THERAPEUTIC IMPACT OF CORTICOSTEROIDS
Multiple adverse reactions potentially related to steroid therapy reported by several studies are: gastroduodenal ulcers, infection, delayed wound healing, diabetes decompensation, neuromyopathy. However, although steroids high-dose remains associated with an increase of nosocomial infections, recent trials involving a low-dose hydrocortisone treatment reveal contradictory data. In fact, whereas the results reported by Annane et al reveal a decrease in infectious risks, CORTICUS study reveals an increased risk 1.27 (95% CI, 0.96-1.68). In this study, a recurrence of the shock and initial infection seemed more common for patients receiving corticosteroids [OR: 1.37 (1.05 to 1.79)].
On the one hand, these results could be explained by the use of corticosteroids at anti-inflammatory doses; on the other hand, we may think they are related to the high prevalence of inadequate empirical antibiotic therapy -rather than the specific effect of glucocorticoids. Moreover, the risk of hyperglycemia was 1.18 (95% CI, 1.07-1.31).53
Although, these data must be confirmed by other studies, it’s important to mention that several studies have shown a tendency to hyperglycemia, more pronounced among patients treated with corticosteroids. It is thus recommended to get adequate glycemic control with insulin therapy, regardless of the chosen administration regimen.
Some authors59 are also in favor of a continuous infusion of corticosteroids instead of sequential administration of successive bolus, to allow improved glycemic control. It may be noted that, a study reported an increased incidence of hypernatremia among patients treated with hydrocortisone, but without real clinical consequence.
In light of this multitude inconsistent data some time with high controversy, it is clear that the use of early high-dose corticosteroids is not only not helpful, but seems to be potentially dangerous for patients with severe sepsis and septic shock.7,8,9,57
Indeed, results from multiple controlled clinical trials clearly showed that low-dose corticosteroid replacement therapy in septic shock is associated with improved blood pressure. It is also mentioned an improvement in terms of shorter duration of vasopressor support in patients with septic shock.7,8,44,46,47,49,51,57
However, on the basis of these data, it is absolutely not possible to mention any advantage for survival among patients with vasopressor-dependent septic shock.
In the same frame, we see that the results of recent studies do not provide a sharp answer to the question of whether there is an improvement in all-cause 28-day mortality rate associated with the use of low-dose hydrocortisone replacement therapy in patients with vasopressor -dependent septic shock.49,53
Moreover, Fludrocortisones does not seem to be a necessary adjuvant therapy. It could be associated with an increased risk of infection.
Thus, according to current recommendations, it is appropriate to reserve the use of corticosteroids in patients with septic shock (not severe sepsis) and whose hemodynamic remains unstable despite adequate fluid resuscitation and insufficiently responsive to administration vasopressor.
No catecholamine threshold is set by the authors to define "inadequate blood pressure response" but experts seem to agree on a dose ≥ 0.5 μg/kg/min adrenaline or noradrenalin. It would not be necessary either to realize an exploration of the HPA axis before considering such a course of action.
Regarding the advocated dose of hydrocortisone, according to the authors, it should be less than 300 mg/day, but they do not reach a decision on the duration of treatment. Literature data recommend treatment of 5 to 7 days if the shock persists.
The schemes proposed by experts are then either 200 mg/d in 4 injections of either a bolus of 100 mg followed by a continuous infusion of 240 mg/day.
It is also recommended to start a gradual decrease in doses at the end of treatment to avoid a rebound of shock: 50 mg / 12 h for 3 days and then 50 mg / 24 h for three days before the full stop.
CONCLUSION
Considering the uncertainty regarding the most suitable strategy and optimal duration of low dose, and the absence of homogeneity of diagnostic criteria and evaluations between the various clinical trials, limiting de facto any attempt to compare, it appears necessary to consider other clinical trial types: prospective, randomized, controlled, and double blind with well-defined upstream inclusion and exclusion criteria.
Only then we may conclude some recommendations regarding the potential benefits, the useful duration, the optimal dose and mode of administration (continuous or intermittent, abrupt cessation or by de-escalation) of corticosteroid therapy of vasopressor-dependent septic shock.
Conflict of interests: None
Authors’ Contribution:
AH: Concept, conduction of the study work, literature search and manuscript editing
ML: Conduction of the study, correction
KL: Literature search and manuscript editing
REFERENCES
- Rossi A, Illouz S, Annane D. Should we still use corticosteroids to treat severe sepsis and septic shock? Réanimation. 2009 June;18(4): 309-322. doi : 10.1016/j.reaurg.2009.03.006.
- Dellinger RP. Steroid therapy of septic shock: the decision is in the eye of the beholder. Crit Care Med. 2008 Jun;36(6):1987–1989.. [PubMed] doi: 10.1097/CCM.0b013e31817d7ee4.
- Sessler CN. Steroids for septic shock: back from the dead? (Con). Chest. 2003 May;123(5 Suppl):482 S–489S. [PubMed]
- Balk RA. Steroids for septic shock: back from the dead? (Pro). Chest. 2003 May;123(5 Suppl):490 S–499S. [PubMed]
- Menon K, Ward RE, Lawson ML, Gaboury I, Hutchison JS, Hébert PC. Canadian Critical Care Trials Group. A prospective multicenter study of adrenal function in critically ill children. Am J Respir Crit Care Med. 2010;182(2):246–251. [PubMed] [Free full text] doi: 10.1164/rccm.200911-1738OC.
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008 Jan;36(1):296–327. [PubMed]
- Minneci PC, Deans KJ, Banks SM, Eichacker PQ, Natanson C. Meta- analysis: the effect of steroids on survival and shock during sepsis depends on the dose. Ann Intern Med. 2004 Jul 6;141(1):47–56. [PubMed] [Free full text]
- Keh D, Sprung CL. Use of corticosteroid therapy in patients with sepsis and septic shock: an evidence-based review. Crit Care Med. 2004 Nov;32(11 Suppl):S527–S533. [PubMed]
- Carlet J. From mega to more reasonable doses of corticosteroids: a decade to recreate hope. Crit Care Med. 1999 Apr;27(4):672–674. [PubMed]
- Matot I, Sprung CL. Corticosteroids in septic shock: resurrection of the last rites? Crit Care Med. 1998 Apr;26(4):627–629. [PubMed]
- Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995 Aug;23(8):1430– 1439. [PubMed]
- Patel GP, Balk RA. Systemic steroids in severe sepsis and septic shock. Am J Respir Crit Care Med. 2012 Jan 15;185(2):133-139. [PubMed] [Free full text] doi: 10.1164/rccm.201011-1897CI.
- Daughaday WH. Binding of corticosteroids by plasma proteins. I. Dialysis equilibrium and renal clearance studies. J Clin Invest. 1956 Dec;35(12):1428—33. [PubMed] [Free full text]
- Annane D, Cavaillon JM. Corticosteroids in sepsis: from bench to bedside? Shock. 2003 Sep;20(3):197—207. [PubMed]
- Melby JC, Spink WW. Comparative studies on adrenal cor- tical function and cortisol metabolism in healthy adults and in patients with shock due to infection. J Clin Invest. 1958 Dec;37(12):1791—8. [PubMed] [Free full text]
- Cooper MS, Bujalska I, Rabbitt E, Walker EA, Bland R, Sheppard MC, et al. Modulation of 11 hydroxysteroid deshydrogenase isozymes by proinflammatory cytokines in osteoblasts: an autocrine switch from glucocorticoid inactivation to activation. J Bone Miner Res 2001;16: 1037—44. [PubMed] [Free full text]
- Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995 May 18;322(20):1351—62. [PubMed]
- Cope CL, Black E. The production rate of cortisol in man. Br Med J. 1958 May 3;1(5078):1020—4. [PubMed] [Free full text]
- Pilkis SJ, Granner DK. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol. 1992;54:885—909. [PubMed]
- Stahn C, Lowenberg M, Hommes DW, Buttergereit F. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol. 2007 Sep 15;275(1-2):71—8. [PubMed]
- Ehrchen J, Steinmüller L, Barczyk K, Tenbrock K, Nacken W, Eisenacher M, et al. Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood. 2007 Feb 1;109(3):1265—74. [PubMed] [Free full text]
- Annane D, Bellissant E. Impact of corticosteroids on the vascular response to catecholamines in septic shock. Réanimation. 2002;11:111–116.
- Bhagat K, Collier J, Vallance P. Local venous responses to endotoxin in humans. Circulation 1996 Aug 1;94(3):490—7. [PubMed] [Free full text]
- Bellissant E, Annane D. Effect of hydrocortisone on phenylephrine: mean arterial pressure dose-response relationship in septic shock. Clin Pharmacol Ther. 2000 Sep;68(3):293—303. [PubMed]
- Sakaue M, Hoffman BB. Glucocorticoids induce transcription and expression of the alpha 1B adrenergic receptor gene in DTT1 MF-2 smooth muscle cells. J Clin Invest. 1991 Aug; 88(2):385—9. [PubMed] [Free full text]
- Hafezi-Moghadam A, Simoncini T, Yang Z, Lambourg FP, Plumier JC, Rebsamen MC, et al. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med. 2002 May;8(5):473—9. [PubMed] [Free full text]
- Mesotten D, Vanhorebeek I, Van den Berghe G. The altered adrenal axis and treatment with glucocorticoids during critical illness. Nat Clin Pract Endocrinol Metab. 2008 Sep;4(9):496–505. [PubMed] [Free full text] doi: 10.1038/ncpendmet0921.
- Jabre P, Combes X, Lapostolle F, Dhaouadi M, Ricard-Hibon A, Vivien B, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomized controlled trial. Lancet. 2009; 374:293–300. [PubMed] doi: 10.1016/S0140-6736(09)60949-1.
- Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003 Feb 20;348(8):727—34. [PubMed]
- Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004 Apr 15;350(16):1629—38. [PubMed]
- Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critical ill adult patients: Consensus statements from an international task force by the American College of Critical Care Medicine.Crit Care Med. 2008 Jun;36(6):1937—49. [PubMed] doi: 10.1097/CCM.0b013e31817603ba.
- Rothwell PM, Udwadia ZF, Lawler PG. Cortisol response to corticotropin and survival in septic shock. Lancet. 1991 Mar 9;337(8741):582—3. [PubMed]
- Lipiner-Friedmann D, Sprung CL, Laterre PF, Weiss Y, Goodman SV, Vogeser M, et al. Adrenal function in sepsis: The restrospective corticus cohort study. Crit Care Med. 2007 Apr;35(4):1012—8. [PubMed]
- Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critical ill adult patients: Consensus statements from an international task force by the American College of Critical Care Medicine.Crit Care Med. 2008 Jun;36(6):1937—49. [PubMed] doi: 10.1097/CCM.0b013e31817603ba.
- Prigent H, Maxime V, Annane D. Science review: mechanisms of impaired adrenal function in sepsis and molecular actions of glucocorticoids. Crit Care. 2004 Aug;8(4):243—52. [PubMed] [Free full text]
- Salgado DR, Verdeal JC, Rocco JR. Adrenal function testing in patients with septic shock. Crit Care. 2006;10(5):R149. [PubMed] [Free full text]
- Siraux V, De Backer D, Yalavatti G, Melot C, Gervy C, Mockel J, et al. Relative adrenal insufficiency in patients with septic shock: comparison of low-dose and conventional corticotropin tests. Crit Care Med. 2005 Nov;33(11):2479–2486. [PubMed]
- Arafah BM. Review: hypothalamic pituitary adrenal function during critical illness: limitations of current assessment methods. J Clin Endocrinol Metab. 2006 Oct;91(10):3725–3745. [PubMed] [Free full text]
- Annane D, Sébille V, Troché G, Raphael JC, Gajdos P, Bellissant E. A 3-level prognostic clas- sification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000 Feb 23;283(8):1038–1045. [PubMed]
- McGee S, Hirschmann J. Use of corticosteroids in treating infectious diseases. Arch Intern Med. 2008 May 26;168(10):1034–1046. [PubMed] doi: 10.1001/archinte.168.10.1034.
- Schumer W. Steroids in the treatment of clinical septic shock. Ann Surg. 1976 Sep;184(3):333–341. [PubMed] [Free full text]
- Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987 Sep 10;317(11): 653–658. [PubMed]
- Veterans Administration Systemic Sepsis Cooperative Study Group. Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med. 1987 Sep 10;317(11):659–665. [PubMed]
- Sprung CL, Caralis PV, Marcial EH, Pierce M, Gelbard MA, Long WM, et al. The effects of high-dose corticosteroids in patients with septic shock: a prospective, controlled study. N Engl J Med. 1984 Nov 1;311(18):1137–1143. [PubMed]
- Sessler CN. Steroids for septic shock: back from the dead? (Con). Chest. 2003 May;123(5 Suppl):482S–489S. [PubMed]
- Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998 Apr;26(4):645–650. [PubMed]
- Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, et al. Stess doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med. 1999 Apr;27(4):723–732. [PubMed]
- Yildiz O, Doganay M, Aygen B, Guven M, Kelestimur S, Tutuu A. Physiological-dose steroid therapy in sepsis. Crit Care. 2002 Jun;6(3):251– 259. [PubMed] [Free full text]
- Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisones on mortality in patients with septic shock. JAMA. 2002 Aug 21;288(7):862–871. [PubMed]
- Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving sepsis campaign guidelines for severe sepsis and septic shock. Crit Care Med. 2004 Mar;32(3):858–873. [PubMed]
- Oppert M, Schindler R, Husung C, Offermann K, Graf KJ, Boenisch O, Barckow D, Frei U, Eckardt KU. Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit Care Med. 2005 Nov;33(11):2457–2464. [PubMed]
- Moran JL, Graham PL, Rockliff S, Bersten AD. Updating the evidence for the role of corticosteroids in severe sepsis and septic shock: a Bayesian meta-analytic perspective.Crit Care. 2010;14(4):R134. [PubMed] [Free full text] doi: 10.1186/cc9182
- Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008 Jan 10;358(2):111–124. [PubMed] doi: 10.1056/NEJMoa071366.
- Cuthbertson BH, Sprung CL, Annane D, Chevret S, Garfield M, Goodman S, et al. The effects of etomidate on adrenal responsiveness and mortality in patients with septic shock. Intensive Care Med. 2009 Nov;35(11):1868–1876. [PubMed] doi: 10.1007/s00134-009-1603-4.
- Annane D. Corticosteroids for sepsis: registry versus Cochrane systematic review! Crit Care. 2010;14(4):185. [PubMed] [Free full text] doi: 10.1186/cc9188
- Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995 Aug ;23(8):1430– 1439. [PubMed]
- Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De Gaudio R, et al. Cortico- steroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009 Jun 10;301(22):2362–2375. [PubMed] doi: 10.1001/jama.2009.815
- Russell JA, Walley KR, Gordon AC, Cooper DJ, Herbert PC, Singer J, et al. Interaction of vasopressin infusion, corticosteroid treatment, and mortality of septic shock. Crit Care Med. 2009 Mar;37(3):811–818. [PubMed] doi: 10.1097/CCM.0b013e3181961ace.
- Loisa P, Parviainen I, Tenhunen J, Hovilehto S, Ruokonen E. Effect of mode of hydrocortisone administration on glycemic control in patients with septic shock: a prospective randomized trial. Crit Care. 2007;11(1):R21. [PubMed] [Free full text]
- Veterans Administration Systemic Sepsis Cooperative Study Group. Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med. 1987 Sep 10;317(11): 659-665. [PubMed]
- Cicarelli DD, Vieira JE, Bensenor FE. Early dexamethasone treatment for septic shock patients: a prospective randomized clinical trial. Sao Paulo Med J. 2007 Jul 5;125(4):237-241. [PubMed] [Free full text]
- Chawla K, Kupfer Y, Tessler S. Hydrocortisone reverses refractory septic shock. Crit Care Med. 1999;27:A33. doi: 10.1097/00003246-199901001-00022.