Delima Radzwa Hasan 1 , Wan Mohd Nazaruddin Wan Hassan 2 , Mohamad Hasyizan Hassan 3 , Rhendra Hardy Mohamad Zaini 4 , Sanihah Che Omar 5
Authors affiliations:
Department of Anesthesiology and Intensive Care, School of Medical Sciences & Hospital Universiti Sains Malaysia, Universiti Sains Malaysia, 16150 Kubang Kerian, Kota Bharu, Kelantan, Malaysia.
Abstract
Background: This study aimed to compare the sustainability of recording between cerebral state index (CSI) and bispectral index (BIS) monitors during supratentorial craniotomy.
Methodology: A total of 42 patients for elective supratentorial craniotomy, aged 18–60 y, ASA I–II, were randomized into two groups: Group CSI (n = 21) and Group BIS (n = 21). All patients underwent surgery under the target-controlled infusion technique for propofol and remifentanil. CSI and BIS sensors were applied accordingly over the forehead before induction, and the index was continuously recorded. Anesthesia was maintained between a range of 40 and 60 in the index. The percentage and time of sustainable recording, and association with causes of unsustainable recording were documented.
Results: There were no significant differences in the percentage of sustainable monitoring (66.7% vs. 71.4%; P = 0.739) and mean duration of sustainable monitoring (437.2 ± 221.8 vs. 407.3 ± 174.6 min; P = 0.631) between CSI and BIS. The causes of recording interruption were comparable between the two groups. Within the BIS group, there was a significant association between sustained recording and surgical incision site (p = 0.012) with the most sustainable recording was a frontoparietal incision (73.3%).
Conclusion: CSI and BIS monitors were comparable in their ability to sustain a recording of index during supratentorial craniotomy.
Key words: Intravenous, anesthesia; Cerebral state index monitor; Bispectral index; Neurosurgery; Craniotomy
Citation: Hasan DR, Hassan WMNW, Hassan MH, Zaini RHM, Omar SC. A comparison of sustainable recording between cerebral state index (CSI) and bispectral index (BIS) monitors during total intravenous anesthesia using target-controlled infusion technique for elective supratentorial craniotomy. Anaesth. pain intensive care 2021;25(6):763–770 ; DOI: 10.35975/apic.v25i6.1700
Received: April 15, 2021, Reviewed: October 3, 2021, Accepted: October 20, 2021
Introduction
Total intravenous anesthesia (TIVA) is a technique of anesthesia that is commonly chosen for neuroanesthesia, particularly in poor intracranial compliance conditions. The cerebral vasoconstrictive nature of intravenous (IV) anesthetic agents causes a reduction of cerebral blood flow-cerebral metabolism coupling mechanism as well as intracranial pressure (ICP). The right technique, together with the appropriate choice of pharmacotherapy, are the key principles for a good outcome during neuroanesthesia. In recent years, TIVA using the target-controlled infusion (TCI) technique has emerged as a standard method for neurosurgical patients because of its titrability and wider options of pharmacokinetic models of main drugs, such as propofol and remifentanil. A combination of electroencephalogram (EEG)-derived monitors, such as bispectral index (BIS) monitors during TIVA, also allows the titration and maintenance of an adequate depth of anesthesia.1 A study of patients with severe traumatic brain injury showed that TIVA with propofol-based infusion provided better brain relaxation and maintained normal ICP and better hemodynamics when compared with inhalational anesthesia using isoflurane at less than 1 minimum alveolar concentration (MAC).2
The TIVA technique is previously assumed to be more related to the risk of intraoperative awareness than inhalational anesthesia because of its limitation in monitoring the depth of anesthesia. Inhalational anesthesia uses MAC value to guide anesthetic depth, but there is previously no such drug concentration monitoring in TIVA until the availability of TCI. Therefore, monitors that can measure the depth of anesthesia, such as EEG-based monitoring devices, are helpful during TIVA to reduce intraoperative drug consumption as well as intraoperative awareness.3 A prospective observational study that evaluated the incidence and characteristics of awareness with recall (AWR) during general anesthesia showed that the overall incidence of AWR was 1.0% (39/3921 patients). Anesthesia techniques without halogenated inhalational agents showed a higher incidence of AWR, which was 1.1% in TIVA-propofol, 0.59% in balanced anesthesia, 5.0% in O2/N2O-based anesthesia and 0.9% in other anesthetic techniques, which were mainly propofol boluses for short procedures.4
There are various types of EEG-derived monitoring devices that are used to monitor the depth of anesthesia, and among the established devices is the BIS monitor. It is a quantitative electroencephalographic device that is widely used to assess the hypnotic component of anesthesia, and a level between 40 and 60 is recommended for an adequate level of the hypnotic state. However, the use of BIS during neurosurgery is challenging because of the proximity of the forehead sensor to the surgical site. There are high possibilities of interruption of BIS recording due to contamination of the forehead sensor with blood or antiseptic cleaning solution. At the same time, the design and size of a BIS forehead sensor in the form of a long strip can also be interfered with the site of surgical incision, the sites of the Mayfield skull clamp and the position of the head.

The availability of another EEG-derived device, the cerebral state index (CSI) monitor, has the potential to reduce the interruption of monitoring because of its smaller sensors, and the device only requires two points of separate sensor placements over the forehead. A study has shown that during sevoflurane-nitrous oxide anesthesia, the CSI was not significantly different from the BIS in the awake state and with a sevoflurane concentration of 0.5–1.5%.5 We always encounter the problems of interruption of BIS monitoring during neuroanesthesia, but there is no study comparing this issue between the two devices. Therefore, this study aimed to compare the sustainability of recording and identify the potential causes of interruption between these two devices during neurosurgery under TIVA-TCI.
Methodology
It was a prospective, single-blinded, randomized-controlled trial, which was conducted after approval from the Institutional Ethics Committee (approval code: USM/JEPeM:17030190) and written consent from the patients. The inclusion criteria were patients who were scheduled for elective supratentorial craniotomy with a Glasgow Coma Scale of 15, aged between 18 and 65 y and in American Society of Anesthesiologists (ASA) classes I and II. Patients were excluded from the study if they were pregnant or known to be allergic to the studied drugs or if the type of surgery was bifrontal craniotomy. Patients were withdrawn from the study if they were unstable intraoperatively due to hemodynamic instability or intraoperative complications.

The randomization was computer-generated, and the sequence of randomization was concealed in the opaque envelope until it was opened in the morning of the surgery by the anesthesiology in charge. After eligibility screening during preoperative review, 42 patients were selected and randomized into two groups: Group CSI (n = 21) and Group BIS (n = 21) (Figure 1).
Upon arrival at the operation theatre, standard monitors were applied to all patients, including non-invasive blood pressure, electrocardiogram, capnography and pulse oximetry. Two large bore IV branulas were inserted before induction, and one of them was dedicated to TIVA-TCI.
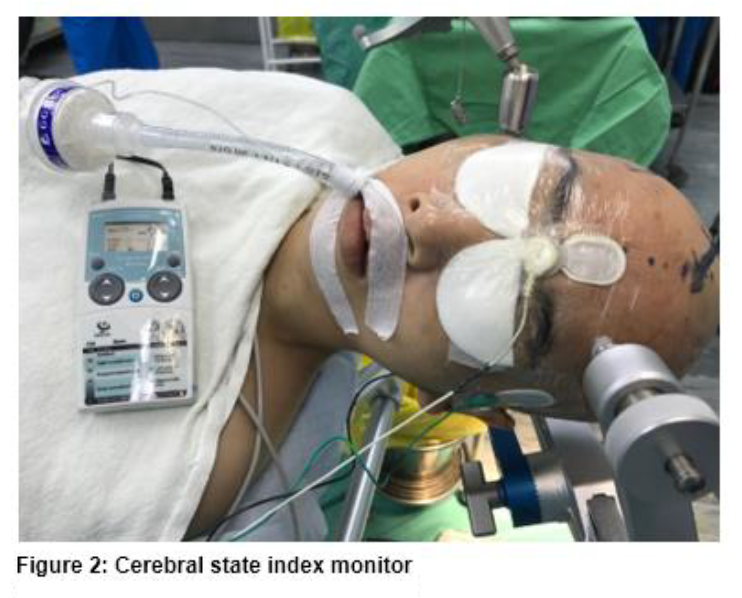
The skin was prepped with alcohol wipes, and sensors of depth of anesthesia monitoring devices were applied according to manufacturer guidelines. Group CSI was applied with CSI sensors at three points, which were at the middle of the forehead, the lateral part of the forehead and the mastoid. CSI sensors were small rounded sensors that were independent from each other. After that, the cable was connected to a Cerebral State Monitor (CSM) M2 (Danmeter, Odense, Denmark) (Figure 2).
Group BIS was applied with a BIS sensor in the form of a long strip with four points of electrodes at the middle of the forehead, above the orbital wall, in between the middle of the forehead and the orbital wall electrodes, and at the bony area of the corner of the eyeball. The cable was subsequently connected to a Bispectral index monitor, BISTM Quatro XP (Covidien, Dublin, Ireland) (Figure 3).
The baseline values of the index were recorded in both groups before induction. In both types of sensors, Tegaderm™ transparent film dressing was applied on top of the sensors to provide a waterproof, sterile barrier to external contaminants during surgery.
Both groups received TCI mode of TIVA using two TCI pumps, Injectomat® TIVA Agilia (Frasenius Kabi, Bad Homburg, Germany). All patients were induced with TCI remifentanil at a target effect concentration of 2.0 ng ml-1 and TCI propofol at a target plasma concentration of 4 µg ml-1. If patients were still conscious after 3 min, TCI propofol was increased by 1 µg ml-1 every 1 min until successful induction, which was characterized by loss of eyelash reflex and loss of verbal reflex. Both indexes at successful induction were recorded for both groups. After a successful induction, IV rocuronium 0.6 mg/kg was given for muscle relaxation, and TCI remifentanil was increased to 4 ng ml-1 for obtunding sympathetic reflexes during intubation. The arterial line, central venous line and central bladder catheter were inserted after induction. Anesthesia was maintained with TCI propofol 3-6 µg/ml and TCI remifentanil 2-8 ng/ml to achieve the target of either BIS or CSI of 40 to 60. CSI and BIS recording were monitored throughout the surgery, and if any interruption of recording occurred, the causes of interruption and the duration of sustainable recording were documented. The duration of sustainable recording was defined as the time from baseline index to interruption or until the completion of surgical closure was recorded. All patients were brought to the neurosurgical intensive care unit for post-operative stabilization and early extubation there.



Sample size was calculated using Power and Sample Size Calculations version 3.0.10 (January 2009, © 1997–2009 by William D. DuPont and Walton D. Plummer) based on a previous study by Denman et al.6 with a mean difference of 8.60 ± 9.08, power of 0.8 and type I error of 0.05. Based on the calculation, 19 subjects were required in each group, and after considering the 10% that dropped out, the total sample size was 42 patients.
Data was analyzed using Statistical Package for the Social Sciences (SPSS) software version 24.0 (SPSS Inc., USA). Categorical data were analyzed with either chi-square or Fisher’s exact test, and numerical data were analyzed with either an independent t-test or Mann-Whitney test. P < 0.05 was considered a significant difference.
Results
A total of 42 patients were enrolled in this study, with 21 patients in each group. There were no significant differences in demographic data between groups. The percentages of head position, Mayfield skull clamp position and site of skin incision were also comparable (Table 1).
There was no significant difference in the mean CSI and BIS at baseline (93.4 ± 4.0 vs. 93.1 ± 6.1; p = 0.859) or after a successful induction (47.6 ± 13.1 vs. 45.8 ± 12.0; p = 0.652). There was also no significant difference in the percentage of sustainable recording (66.7% vs. 71.4%; p = 0.739) and mean duration of sustainable recording (437.2 ± 221.8 vs. 407.3 ± 174.6 min; p = 0.631). The causes of recording interruption were also comparable between the two groups (Table 2).
The association of sustainable recording and skin incision site was significant within the BIS group (P = 0.012). The highest percentage of sustainable recording was in the frontoparietal incision (73.3%), and the highest percentage of recording interruption was in the parietooccipital incision (50%). There were no other significant associations within the BIS group and the CSI group (Table 3).
Discussion
The basis of our study was to determine the options of EEG-derived depth of anesthesia monitoring, which was able to sustain recording throughout neurosurgery even though with some potential problems, such as risk of contamination, proximity of forehead sensor to the surgical site, position of the head during surgery and position of Mayfield skull clamp. Our result showed that the percentage of sustainable monitoring and mean duration of sustainable monitoring between CSI and BIS were comparable. The causes of recording interruption were comparable between the two groups. The only significant finding was an association between sustainable recording and surgical site within the BIS group.
The index values between CSI and BIS at baseline (93.4 ± 4.0 vs. 93.1 ± 6.1) and induction (47.6 ± 13.1 vs. 45.8 ± 12.0) in our study were comparable. These results were similar to a few other studies comparing CSI and BIS. Zhong et al. compared the performance of the CSI to BIS during TCI of propofol in 20 patients and found that in spite of larger baseline variation, CSI performance was equally well with BIS in terms of prediction probability values. CSI also had a good correlation with the level of sedation.7 Anderson et al. conducted a study to determine the degree of agreement between the two monitors during anesthesia for day care surgery, and their result showed that CSI and BIS had similar patterns and numerical values but with occasional large discrepancies between pair-wise readings.8 Pilge et al. compared CSI and BIS during propofol–remifentanil anesthesia, and the result showed that 87% of patients demonstrated good fit between the indexes. In seven of their patients, they determined major deviations of index between the two in which CSI indicated that in the parts of the course of anesthesia, CSI showed the patients were awake despite clinical sleep, but BIS identified it correctly.9 There was a time delay of between 14 and 155 s found in both devices, but these delays were not constant.10
To the best of our knowledge, no previous study has been conducted to evaluate the sustainability of recording between CSI and BIS throughout neurosurgical anesthesia. The use of the TIVA-TCI technique during most of our elective neurosurgery required EEG-derived depth of anesthesia monitoring to prevent intraoperative awareness and as a guide to drug titration. The EEG-derived monitor that we commonly use during neurosurgery is a BIS monitor. However, based on our clinical experience, interruptions of recording occurred along the way of the surgery either because of contamination of the sensor by an antiseptic cleaning solution or by blood. There were sometimes unintentional disturbances of sensor skin contact by the surgeons because of sharing the surgical field and placement site with the surgery. The other possibilities that might interfere with the recording are the site of the surgical incision, the position of the Mayfield skull clamp and the position of the head during surgery. In terms of the design of the sensor, the CSI sensor appears smaller and consists of three separate sensors, which are only two of the sensors that need to be placed over the forehead at the middle and side of the forehead and the one over the mastoid. These features are potentially better to sustain the recording than the BIS sensor with potentially lesser exposure to contamination during surgery. If we look at the BIS sensor, it is designed in a long strip with three points of connection over the forehead, with another one at the corner of the eyeball. The width of the strip is also bigger than that of the CSI sensor. This design occupies more space over the forehead, and the potential of exposure to contamination is higher. In terms of the design of the monitor, the CSI monitor is more compact and portable.
In our study, both BIS and CSI were able to sustain their recording equally well even though, by percentage, CSI recorded a lower value than BIS (66.7% vs. 71.4%). In terms of the duration of sustainable monitoring, both devices showed comparable duration even though the CSI duration was slightly longer (437.2 ± 221.8 vs. 407.3 ± 174.6 min). In general, the interruption of recording occurred in one-third of the patients. The commonest cause of unsustainable recording in our study was poor contact of the sensors with the skin, where 85.7% was recorded in the CSI group and 66.7% was in the BIS group. Contamination of the sensors was another cause of unsustainable recording, which was more common in the BIS group (33.3%) than in the CSI group (14.3%). There is an option of using ECG electrodes for CSI to improve the contact of CSI sensors in a future study. Anderson et al. demonstrated that the use of ECG electrode was highly correlated with the CSI obtained using proprietary CSM sensors, saving the cost of the sensor by 90%.11 Even though the comparison between the two groups was not significant, the result showed that CSI had a higher percentage of poor contact, whereas BIS showed a higher percentage of contamination. Within the BIS group, there was a significant association between sustainable recording and the surgical site, whereby the highest site of sustained recording was in the frontoparietal incision (73.3%) and the highest site for unsustainable recording was in the parietooccipital incision (50%). The challenges of using the depth of monitoring sensors over the recommended site at the forehead during supratentorial craniotomy have been addressed in some previous studies. Sharma et al. determined the feasibility of the modification of entropy sensors to a new version of three separated electrodes, such as CSI sensor, in 20 consecutive patients undergoing orbitozygomatic craniotomy and bifrontal craniotomy. The previous version of the entropy sensor was the same concept as the BIS sensor, where three electrodes were placed on one strip of sensor. The result showed that new entropy sensors were more flexible and that entropy value monitoring was possible in all the patients with good correlation to the clinical indices of depth of anesthesia.12
Other than a modification of the sensor, there were a number of studies that looked into alternative placements for the BIS sensor for neurosurgical or maxillofacial procedures. Alternative sensor placements include occipital, post-auricular, auricular, infra-nasal and mandibular. Of these five alternative placements, the occipital area was quite popular. Shiraishi et al. compared BIS sensor placed at the frontal area with sensor placed at the occipital area simultaneously in 25 patients who underwent neurosurgery. They found a strong correlation between these two areas and suggested that the use of the BIS sensor on the occipital area may be valid if required during frontal neurosurgical procedures13. Another study by Dahaba et al. also compared the placement of a BIS occipital sensor, which was considered an off-label placement, with a conventional frontal sensor using the new BIS-Vista™ monitor. The result demonstrated that the agreement of the BIS between two placements was too wide to allow data of the two to be used interchangeably with a variation of anesthetic depth. They concluded that an off-label occipital sensor might be helpful in following a trend of propofol-remifentanil anesthesia in individual cases where frontal access is particularly difficult.14 Another study on alternative sites was conducted in the post-auricular area. Akavipat et al. studied 34 patients who underwent neurosurgery using BIS recorded simultaneously from sensors placed on the frontal and post-auricular areas. They concluded that post-auricular placement of a BIS electrode is a practical alternative to frontal lobe placement. Nevertheless, they still highlighted that proper electrode location was important to minimize error.15 Nelson et al. compared another alternative BIS sensor placement across the nasal dorsum with frontal placement. They determined that the nasal montage produced values that had slightly more variability compared with those ideally desired, but the variability was not clinically significant. In cases where the standard BIS-vista montage would interfere with the operative field, an alternative positioning of the BIS montage across the nasal bridge and under the eye could be used.16
Conclusion
CSI and BIS monitors were comparable in their ability to sustain the recording of index during supratentorial craniotomy.
Acknowledgement
We would like to thank Universiti Sains Malaysia for awarding the short-term research grant (304/PPSP/6315094) for this research.
Conflict of Interest
Authors declare no conflict of interest.
Author’s Contribution
References
Authors affiliations:
Department of Anesthesiology and Intensive Care, School of Medical Sciences & Hospital Universiti Sains Malaysia, Universiti Sains Malaysia, 16150 Kubang Kerian, Kota Bharu, Kelantan, Malaysia.
- {ORCID:0000-0002-4878-1796}; 2. {ORCID:0000-0002-3771-0327}; 3. {ORCID:0000-0001-7264-7549};
- {ORCID:0000-0002-9903-9073}; 5. {ORCID:0000-0002-2483-0784}
Abstract
Background: This study aimed to compare the sustainability of recording between cerebral state index (CSI) and bispectral index (BIS) monitors during supratentorial craniotomy.
Methodology: A total of 42 patients for elective supratentorial craniotomy, aged 18–60 y, ASA I–II, were randomized into two groups: Group CSI (n = 21) and Group BIS (n = 21). All patients underwent surgery under the target-controlled infusion technique for propofol and remifentanil. CSI and BIS sensors were applied accordingly over the forehead before induction, and the index was continuously recorded. Anesthesia was maintained between a range of 40 and 60 in the index. The percentage and time of sustainable recording, and association with causes of unsustainable recording were documented.
Results: There were no significant differences in the percentage of sustainable monitoring (66.7% vs. 71.4%; P = 0.739) and mean duration of sustainable monitoring (437.2 ± 221.8 vs. 407.3 ± 174.6 min; P = 0.631) between CSI and BIS. The causes of recording interruption were comparable between the two groups. Within the BIS group, there was a significant association between sustained recording and surgical incision site (p = 0.012) with the most sustainable recording was a frontoparietal incision (73.3%).
Conclusion: CSI and BIS monitors were comparable in their ability to sustain a recording of index during supratentorial craniotomy.
Key words: Intravenous, anesthesia; Cerebral state index monitor; Bispectral index; Neurosurgery; Craniotomy
Citation: Hasan DR, Hassan WMNW, Hassan MH, Zaini RHM, Omar SC. A comparison of sustainable recording between cerebral state index (CSI) and bispectral index (BIS) monitors during total intravenous anesthesia using target-controlled infusion technique for elective supratentorial craniotomy. Anaesth. pain intensive care 2021;25(6):763–770 ; DOI: 10.35975/apic.v25i6.1700
Received: April 15, 2021, Reviewed: October 3, 2021, Accepted: October 20, 2021
Introduction
Total intravenous anesthesia (TIVA) is a technique of anesthesia that is commonly chosen for neuroanesthesia, particularly in poor intracranial compliance conditions. The cerebral vasoconstrictive nature of intravenous (IV) anesthetic agents causes a reduction of cerebral blood flow-cerebral metabolism coupling mechanism as well as intracranial pressure (ICP). The right technique, together with the appropriate choice of pharmacotherapy, are the key principles for a good outcome during neuroanesthesia. In recent years, TIVA using the target-controlled infusion (TCI) technique has emerged as a standard method for neurosurgical patients because of its titrability and wider options of pharmacokinetic models of main drugs, such as propofol and remifentanil. A combination of electroencephalogram (EEG)-derived monitors, such as bispectral index (BIS) monitors during TIVA, also allows the titration and maintenance of an adequate depth of anesthesia.1 A study of patients with severe traumatic brain injury showed that TIVA with propofol-based infusion provided better brain relaxation and maintained normal ICP and better hemodynamics when compared with inhalational anesthesia using isoflurane at less than 1 minimum alveolar concentration (MAC).2
The TIVA technique is previously assumed to be more related to the risk of intraoperative awareness than inhalational anesthesia because of its limitation in monitoring the depth of anesthesia. Inhalational anesthesia uses MAC value to guide anesthetic depth, but there is previously no such drug concentration monitoring in TIVA until the availability of TCI. Therefore, monitors that can measure the depth of anesthesia, such as EEG-based monitoring devices, are helpful during TIVA to reduce intraoperative drug consumption as well as intraoperative awareness.3 A prospective observational study that evaluated the incidence and characteristics of awareness with recall (AWR) during general anesthesia showed that the overall incidence of AWR was 1.0% (39/3921 patients). Anesthesia techniques without halogenated inhalational agents showed a higher incidence of AWR, which was 1.1% in TIVA-propofol, 0.59% in balanced anesthesia, 5.0% in O2/N2O-based anesthesia and 0.9% in other anesthetic techniques, which were mainly propofol boluses for short procedures.4
There are various types of EEG-derived monitoring devices that are used to monitor the depth of anesthesia, and among the established devices is the BIS monitor. It is a quantitative electroencephalographic device that is widely used to assess the hypnotic component of anesthesia, and a level between 40 and 60 is recommended for an adequate level of the hypnotic state. However, the use of BIS during neurosurgery is challenging because of the proximity of the forehead sensor to the surgical site. There are high possibilities of interruption of BIS recording due to contamination of the forehead sensor with blood or antiseptic cleaning solution. At the same time, the design and size of a BIS forehead sensor in the form of a long strip can also be interfered with the site of surgical incision, the sites of the Mayfield skull clamp and the position of the head.

The availability of another EEG-derived device, the cerebral state index (CSI) monitor, has the potential to reduce the interruption of monitoring because of its smaller sensors, and the device only requires two points of separate sensor placements over the forehead. A study has shown that during sevoflurane-nitrous oxide anesthesia, the CSI was not significantly different from the BIS in the awake state and with a sevoflurane concentration of 0.5–1.5%.5 We always encounter the problems of interruption of BIS monitoring during neuroanesthesia, but there is no study comparing this issue between the two devices. Therefore, this study aimed to compare the sustainability of recording and identify the potential causes of interruption between these two devices during neurosurgery under TIVA-TCI.
Methodology
It was a prospective, single-blinded, randomized-controlled trial, which was conducted after approval from the Institutional Ethics Committee (approval code: USM/JEPeM:17030190) and written consent from the patients. The inclusion criteria were patients who were scheduled for elective supratentorial craniotomy with a Glasgow Coma Scale of 15, aged between 18 and 65 y and in American Society of Anesthesiologists (ASA) classes I and II. Patients were excluded from the study if they were pregnant or known to be allergic to the studied drugs or if the type of surgery was bifrontal craniotomy. Patients were withdrawn from the study if they were unstable intraoperatively due to hemodynamic instability or intraoperative complications.


The randomization was computer-generated, and the sequence of randomization was concealed in the opaque envelope until it was opened in the morning of the surgery by the anesthesiology in charge. After eligibility screening during preoperative review, 42 patients were selected and randomized into two groups: Group CSI (n = 21) and Group BIS (n = 21) (Figure 1).
Upon arrival at the operation theatre, standard monitors were applied to all patients, including non-invasive blood pressure, electrocardiogram, capnography and pulse oximetry. Two large bore IV branulas were inserted before induction, and one of them was dedicated to TIVA-TCI.
The skin was prepped with alcohol wipes, and sensors of depth of anesthesia monitoring devices were applied according to manufacturer guidelines. Group CSI was applied with CSI sensors at three points, which were at the middle of the forehead, the lateral part of the forehead and the mastoid. CSI sensors were small rounded sensors that were independent from each other. After that, the cable was connected to a Cerebral State Monitor (CSM) M2 (Danmeter, Odense, Denmark) (Figure 2).
Group BIS was applied with a BIS sensor in the form of a long strip with four points of electrodes at the middle of the forehead, above the orbital wall, in between the middle of the forehead and the orbital wall electrodes, and at the bony area of the corner of the eyeball. The cable was subsequently connected to a Bispectral index monitor, BISTM Quatro XP (Covidien, Dublin, Ireland) (Figure 3).
The baseline values of the index were recorded in both groups before induction. In both types of sensors, Tegaderm™ transparent film dressing was applied on top of the sensors to provide a waterproof, sterile barrier to external contaminants during surgery.
Both groups received TCI mode of TIVA using two TCI pumps, Injectomat® TIVA Agilia (Frasenius Kabi, Bad Homburg, Germany). All patients were induced with TCI remifentanil at a target effect concentration of 2.0 ng ml-1 and TCI propofol at a target plasma concentration of 4 µg ml-1. If patients were still conscious after 3 min, TCI propofol was increased by 1 µg ml-1 every 1 min until successful induction, which was characterized by loss of eyelash reflex and loss of verbal reflex. Both indexes at successful induction were recorded for both groups. After a successful induction, IV rocuronium 0.6 mg/kg was given for muscle relaxation, and TCI remifentanil was increased to 4 ng ml-1 for obtunding sympathetic reflexes during intubation. The arterial line, central venous line and central bladder catheter were inserted after induction. Anesthesia was maintained with TCI propofol 3-6 µg/ml and TCI remifentanil 2-8 ng/ml to achieve the target of either BIS or CSI of 40 to 60. CSI and BIS recording were monitored throughout the surgery, and if any interruption of recording occurred, the causes of interruption and the duration of sustainable recording were documented. The duration of sustainable recording was defined as the time from baseline index to interruption or until the completion of surgical closure was recorded. All patients were brought to the neurosurgical intensive care unit for post-operative stabilization and early extubation there.



Sample size was calculated using Power and Sample Size Calculations version 3.0.10 (January 2009, © 1997–2009 by William D. DuPont and Walton D. Plummer) based on a previous study by Denman et al.6 with a mean difference of 8.60 ± 9.08, power of 0.8 and type I error of 0.05. Based on the calculation, 19 subjects were required in each group, and after considering the 10% that dropped out, the total sample size was 42 patients.
Data was analyzed using Statistical Package for the Social Sciences (SPSS) software version 24.0 (SPSS Inc., USA). Categorical data were analyzed with either chi-square or Fisher’s exact test, and numerical data were analyzed with either an independent t-test or Mann-Whitney test. P < 0.05 was considered a significant difference.
Results
A total of 42 patients were enrolled in this study, with 21 patients in each group. There were no significant differences in demographic data between groups. The percentages of head position, Mayfield skull clamp position and site of skin incision were also comparable (Table 1).
There was no significant difference in the mean CSI and BIS at baseline (93.4 ± 4.0 vs. 93.1 ± 6.1; p = 0.859) or after a successful induction (47.6 ± 13.1 vs. 45.8 ± 12.0; p = 0.652). There was also no significant difference in the percentage of sustainable recording (66.7% vs. 71.4%; p = 0.739) and mean duration of sustainable recording (437.2 ± 221.8 vs. 407.3 ± 174.6 min; p = 0.631). The causes of recording interruption were also comparable between the two groups (Table 2).
The association of sustainable recording and skin incision site was significant within the BIS group (P = 0.012). The highest percentage of sustainable recording was in the frontoparietal incision (73.3%), and the highest percentage of recording interruption was in the parietooccipital incision (50%). There were no other significant associations within the BIS group and the CSI group (Table 3).
Discussion
The basis of our study was to determine the options of EEG-derived depth of anesthesia monitoring, which was able to sustain recording throughout neurosurgery even though with some potential problems, such as risk of contamination, proximity of forehead sensor to the surgical site, position of the head during surgery and position of Mayfield skull clamp. Our result showed that the percentage of sustainable monitoring and mean duration of sustainable monitoring between CSI and BIS were comparable. The causes of recording interruption were comparable between the two groups. The only significant finding was an association between sustainable recording and surgical site within the BIS group.
The index values between CSI and BIS at baseline (93.4 ± 4.0 vs. 93.1 ± 6.1) and induction (47.6 ± 13.1 vs. 45.8 ± 12.0) in our study were comparable. These results were similar to a few other studies comparing CSI and BIS. Zhong et al. compared the performance of the CSI to BIS during TCI of propofol in 20 patients and found that in spite of larger baseline variation, CSI performance was equally well with BIS in terms of prediction probability values. CSI also had a good correlation with the level of sedation.7 Anderson et al. conducted a study to determine the degree of agreement between the two monitors during anesthesia for day care surgery, and their result showed that CSI and BIS had similar patterns and numerical values but with occasional large discrepancies between pair-wise readings.8 Pilge et al. compared CSI and BIS during propofol–remifentanil anesthesia, and the result showed that 87% of patients demonstrated good fit between the indexes. In seven of their patients, they determined major deviations of index between the two in which CSI indicated that in the parts of the course of anesthesia, CSI showed the patients were awake despite clinical sleep, but BIS identified it correctly.9 There was a time delay of between 14 and 155 s found in both devices, but these delays were not constant.10
To the best of our knowledge, no previous study has been conducted to evaluate the sustainability of recording between CSI and BIS throughout neurosurgical anesthesia. The use of the TIVA-TCI technique during most of our elective neurosurgery required EEG-derived depth of anesthesia monitoring to prevent intraoperative awareness and as a guide to drug titration. The EEG-derived monitor that we commonly use during neurosurgery is a BIS monitor. However, based on our clinical experience, interruptions of recording occurred along the way of the surgery either because of contamination of the sensor by an antiseptic cleaning solution or by blood. There were sometimes unintentional disturbances of sensor skin contact by the surgeons because of sharing the surgical field and placement site with the surgery. The other possibilities that might interfere with the recording are the site of the surgical incision, the position of the Mayfield skull clamp and the position of the head during surgery. In terms of the design of the sensor, the CSI sensor appears smaller and consists of three separate sensors, which are only two of the sensors that need to be placed over the forehead at the middle and side of the forehead and the one over the mastoid. These features are potentially better to sustain the recording than the BIS sensor with potentially lesser exposure to contamination during surgery. If we look at the BIS sensor, it is designed in a long strip with three points of connection over the forehead, with another one at the corner of the eyeball. The width of the strip is also bigger than that of the CSI sensor. This design occupies more space over the forehead, and the potential of exposure to contamination is higher. In terms of the design of the monitor, the CSI monitor is more compact and portable.
In our study, both BIS and CSI were able to sustain their recording equally well even though, by percentage, CSI recorded a lower value than BIS (66.7% vs. 71.4%). In terms of the duration of sustainable monitoring, both devices showed comparable duration even though the CSI duration was slightly longer (437.2 ± 221.8 vs. 407.3 ± 174.6 min). In general, the interruption of recording occurred in one-third of the patients. The commonest cause of unsustainable recording in our study was poor contact of the sensors with the skin, where 85.7% was recorded in the CSI group and 66.7% was in the BIS group. Contamination of the sensors was another cause of unsustainable recording, which was more common in the BIS group (33.3%) than in the CSI group (14.3%). There is an option of using ECG electrodes for CSI to improve the contact of CSI sensors in a future study. Anderson et al. demonstrated that the use of ECG electrode was highly correlated with the CSI obtained using proprietary CSM sensors, saving the cost of the sensor by 90%.11 Even though the comparison between the two groups was not significant, the result showed that CSI had a higher percentage of poor contact, whereas BIS showed a higher percentage of contamination. Within the BIS group, there was a significant association between sustainable recording and the surgical site, whereby the highest site of sustained recording was in the frontoparietal incision (73.3%) and the highest site for unsustainable recording was in the parietooccipital incision (50%). The challenges of using the depth of monitoring sensors over the recommended site at the forehead during supratentorial craniotomy have been addressed in some previous studies. Sharma et al. determined the feasibility of the modification of entropy sensors to a new version of three separated electrodes, such as CSI sensor, in 20 consecutive patients undergoing orbitozygomatic craniotomy and bifrontal craniotomy. The previous version of the entropy sensor was the same concept as the BIS sensor, where three electrodes were placed on one strip of sensor. The result showed that new entropy sensors were more flexible and that entropy value monitoring was possible in all the patients with good correlation to the clinical indices of depth of anesthesia.12
Other than a modification of the sensor, there were a number of studies that looked into alternative placements for the BIS sensor for neurosurgical or maxillofacial procedures. Alternative sensor placements include occipital, post-auricular, auricular, infra-nasal and mandibular. Of these five alternative placements, the occipital area was quite popular. Shiraishi et al. compared BIS sensor placed at the frontal area with sensor placed at the occipital area simultaneously in 25 patients who underwent neurosurgery. They found a strong correlation between these two areas and suggested that the use of the BIS sensor on the occipital area may be valid if required during frontal neurosurgical procedures13. Another study by Dahaba et al. also compared the placement of a BIS occipital sensor, which was considered an off-label placement, with a conventional frontal sensor using the new BIS-Vista™ monitor. The result demonstrated that the agreement of the BIS between two placements was too wide to allow data of the two to be used interchangeably with a variation of anesthetic depth. They concluded that an off-label occipital sensor might be helpful in following a trend of propofol-remifentanil anesthesia in individual cases where frontal access is particularly difficult.14 Another study on alternative sites was conducted in the post-auricular area. Akavipat et al. studied 34 patients who underwent neurosurgery using BIS recorded simultaneously from sensors placed on the frontal and post-auricular areas. They concluded that post-auricular placement of a BIS electrode is a practical alternative to frontal lobe placement. Nevertheless, they still highlighted that proper electrode location was important to minimize error.15 Nelson et al. compared another alternative BIS sensor placement across the nasal dorsum with frontal placement. They determined that the nasal montage produced values that had slightly more variability compared with those ideally desired, but the variability was not clinically significant. In cases where the standard BIS-vista montage would interfere with the operative field, an alternative positioning of the BIS montage across the nasal bridge and under the eye could be used.16
Conclusion
CSI and BIS monitors were comparable in their ability to sustain the recording of index during supratentorial craniotomy.
Acknowledgement
We would like to thank Universiti Sains Malaysia for awarding the short-term research grant (304/PPSP/6315094) for this research.
Conflict of Interest
Authors declare no conflict of interest.
Author’s Contribution
References
- Magazzeni P, Jochum D, Iohom G, Mekler G, Albuisson E, Bouaziz H. Ultrasound-guided selective versus conventional block of the medial brachial cutaneous and the intercostobrachial nerves: a randomized clinical trial. Reg Anesth Pain Med. 2018 Nov;43(8):832-837. [PubMed] DOI: 1097/AAP.0000000000000823
- Perlas A, Lobo G, Lo N, Brull R, Chan VW, Karkhanis R. Ultrasound-guided supraclavicular block: outcome of 510 consecutive cases. Reg Anesth Pain Med. 2009 Mar-Apr;34(2):171-6. [PubMed] DOI: 1097/AAP.0b013e31819a3f81
- Van Geffen GJ. Ultrasound guided peripheral regional anesthesia what are the limits? Reg Anesth Pain Med. 2010;35(5):E41–E42.
- Sviggum HP, Ahn K, Dilger JA, Smith HM. Needle echogenicity in sonographically guided regional anesthesia: blinded comparison of 4 enhanced needles and validation of visual criteria for evaluation. J Ultrasound Med. 2013 Jan;32(1):143-8. [PubMed] DOI: 7863/jum.2013.32.1.143
- Brookes J, Sondekoppam R, Armstrong K, Uppal V, Dhir S, Terlecki M, et al. Comparative evaluation of the visibility and block characteristics of a stimulating needle and catheter vs an echogenic needle and catheter for sciatic nerve block with a low-frequency ultrasound probe. Br J Anaesth. 2015 Dec;115(6):912-9. [PubMed] DOI: 1093/bja/aev351
- Hocking G, Mitchell CH. Optimizing the safety and practice of ultrasound-guided regional anesthesia: the role of echogenic technology. Curr Opin Anaesthesiol. 2012 Oct;25(5):603-9. [PubMed] DOI: 1097/ACO.0b013e328356b835
- Abbal B, Choquet O, Gourari A, Bouic N, Massone A, Biboulet P, et al. Enhanced visual acuity with echogenic needles in ultrasound-guided axillary brachial plexus block: a randomized, comparative, observer-blinded study. Minerva Anestesiol. 2015 Apr;81(4):369-78. [PubMed]
- Nakagawa K, Kamiya T, Arakawa K, Akiyama S, Sakai K. Objective and subjective comparison of the visibility of three echogenic needles and a nonechogenic needle on older ultrasound devices. Acta Anaesthesiol Taiwan. 2015 Mar;53(1):1-6. [PubMed] DOI: 1016/j.aat.2014.11.004
- Nagpaul SR, Kaur N, Kamming D. Needle to nerve time comparison of four different echogenic ultrasound guided regional anaesthesia nerve block needles. Reg Anesth Pain Med. 2011.