Hina Niraj Gadani, MD, DA1, Naitik B. Patel, MD2, Shobhana C. Gupta, MD3
1Associate Professor; 3Professor & Head
Dept. of Anesthesiology, GMERS Medical College, Civil Hospital Campus, Sector – 12, Near Pathikasharam, Gandhinagar, Gujarat 382012, (India)
2Associate Professor, Dept. of Anesthesiology, Gujarat Adani Institute of Medical Sciences (GAIMS), G.K.General Hospital Campus,, Bhuj, Gujarat 370001, (India)
Correspondence: Dr Hina Niraj Gadani, 1/T, F/1, Haridham Enclave, B/H Raysan Petrol Pump,
Koba-Gandhinagar Highway, Raysan, Gandhinagar, Gujarat-382007 (India); Mobile: +91-8690501339; E-mail: hinagadani@gmail.com
ABSTRACT
Background: Preemptive analgesia establishes effective antinociception before surgery and continuation of this effective analgesic level well into the postoperative period. Butorphanol tartrate and pentazocine lactate are opioid analgesics with mixed agonist-antagonist properties.
Aim: The aim of the present study was to compare the preemptive analgesic effect of butorphanol and pentazocine given by intramuscular (IM) route as a primary outcome. Secondary outcome was to compare hemodynamic parameters and the side effects profile.
Methodology: A comparative randomized, single blind, and prospective clinical study in sixty patients ASA physical status I and II was carried out. Patients were demographically similar. Patients were randomized to receive either a butorphanol injection (Group B) 2 mg (n=30) or pentazocine injection (Group P) 60 mg (n=30) both IM 60 min before surgery. Lower abdominal surgeries under spinal anesthesia were selected. Duration of pain relief was recorded by visual analogue scale (VAS) postoperatively up to 24 h. Sedation was measured with Cook's sedation score system. Patients were observed for any change in vital signs and any other side effect for 24 h. Rescue analgesia in the form of IM diclofenac sodium 75 mg was given when VAS≥3.
Results: Duration of analgesia was up to 18 h in Group P while it was extended in Group B, but this was statistically not significant. Requirements of rescue analgesia were higher and occurred earlier in Group P, although not statistically significant. Sedation score was also comparable. Hemodynamic changes were not significant with the exception of an increase in mean arterial pressure in Group P. No severe side effects were observed in any patient of either group.
Conclusion: Butorphanol a mixed agonist-antagonist opioid in the dose of 2 mg IM is an acceptable alternative to pentazocine as a pre-emptive analgesic due to longer duration of analgesia and greater analgesic efficacy with low incidence of side effects.
Key words: Injection butorphanol tartrate, injection pentazocine lactate, postoperative analgesia, preemptive analgesia.
Citation: Gadani HN, Patel NB, Gupta SC. Role of butorphanol in preemptive analgesia: A comparison with pentazocine. Anaesth, Pain & Intensive Care 2017;21(1):44-51
Received: 12 Sep 2016; Reviewed: 14 Sep 2016, 6 Jan 2017; Corrected: 16 Sep 2016, 17 Jan 2017; Accepted: 4 Feb 2017
INTRODUCTION
Postoperative pain relief can be achieved by many ways, e. g. parenteral, epidural/local blocks or patient controlled analgesia (PCA); but parenteral method emerges as an effective and low cost alternative in resource poor settings.
Preemptive analgesia is a treatment that is initiated before the surgical procedure in order to reduce the sensitization of peripheral and central pain pathways. Due to this protective effect, it is more effective than a similar analgesic treatment initiated after surgery. Immediate postoperative pain may be reduced and the development of chronic pain may be prevented.1
Pure opioid agonists are effective analgesics but under schedule II narcotics with limited access, more side effects and addictive properties. Synthetic opioid agonist-antagonists share the analgesic property of pure agonists with reduced liability of dependence, respiratory depression and other side effects. Butorphanol, Pentazocine and Nalbuphine are synthetic opioid agonist-antagonists.
Butorphanol tartrate is a synthetic analgesic of 3:14 dihydroxymorphinan structure. It exhibits partial agonist and antagonist activity at the μ-opioid receptor as well as partial agonist activity at the kappa (k)-opioid receptor. Stimulation of these receptors on central nervous system neurons causes an intracellular inhibition of adenylate cyclase, closing of influx membrane calcium channels and opening of membrane potassium channels. This leads to hyperpolarization of the cell membrane and suppression of action potential transmission of ascending pain pathways. When given parenterally it is five times as potent as morphine. k-agonism can cause dysphoria at therapeutic or supertherapeutic doses. This gives butorphanol a lower potential for abuse than other opioid drugs. It is not classified as a controlled drug under the misuse of drugs (1971). 2, 3
Pentazocine is a synthetically-prepared prototypical mixed agonist–antagonist opioid analgesic drug of the benzomorphan class of opioids and is used to treat moderate to severe pain. This compound may exist as one of two enantiomers, named (+)-pentazocine and (−)-pentazocine. (−)-pentazocine is a k-opioid receptor agonist, while (+)-pentazocine is not, instead displaying a ten-fold greater affinity for the σ receptor. Pentazocine was introduced in 1964 for its analgesic properties. Internationally pentazocine is a Schedule III drug under the Convention on Psychotropic Substances. 4
The present clinical study compared pre-emptive analgesic effect and side effects of two mixed agonist-antagonist opioids, butorphanol tartrate and pentazocine lactate given by IM route for lower abdominal surgeries under spinal anesthesia. We choose surgeries under spinal anesthesia, as under a spinal anesthetic, patients typically require significantly lower amounts of narcotics, so they emerge from surgery refreshed and alert rather than groggy and wiped out. Studies have shown a lower rate of post-operative complication with regional anesthesia compared to general anesthesia. 5
Reason for conducting this study is to compare preemptive analgesic efficacy and side effects of potent synthetic opioid agonist-antagonists, butorphanol and pentazocine. Butorphanol does not fall under the category of controlled drug; it is easily distributed and stored without special arrangements. We preferred Pentazocine as a reference substance as it is made easily available by hospital authority and its effects are well documented. Access to other recent pure opioid analgesics is limited due to complicated regulations. 6
METHODOLOGY
Approval for the study was taken from institutional ethical committee. A comparative, randomized, single blind, and prospective clinical study in sixty patients of ASA physical status I and II was carried out at GuruGobind Singh Hospital, Jamnagar, over a period of 1 year. Patients having age of 21-50 years and weighing 50-70 kg were included in the study. Lower abdominal surgeries requiring spinal anesthesia were selected. Patients with any systemic disease, psychological disease, on any chronic medication, history of allergy to any drug and pregnant patients were excluded from the study. All patients were educated about use of VAS which was used for assessment of pain postoperatively. A 0-10 cm line was drawn. Point 0 was considered as no pain and point 10 was considered as worst pain ever. A written informed consent was taken from all patients. Patients were randomly divided into two groups by sealed envelope technique, inj. butorphanol tartrate group (Group B) (n=30) and inj. pentazocine lactate group (Group P) (n=30). Group B patients received inj. butorphanol tartrate 2 mg (1 mg/ml) while Group P patients received inj. pentazocine lactate 60 mg (30 mg/ml) IM 60 min before surgery. The nurse who was giving injection, was not blinded to the study drug. The observer remained blinded to the study drug. Multipara monitor was attached. On arrival in the operation room, initial assessment of degree of sedation was performed using Cook’s sedation score (Box 1).7
Before conducting spinal block multipara monitor was attached. For spinal anesthesia inj. Bupivacaine hydrochloride 0.5% heavy 2-2.5 ml was used. Preoperative and intraoperative monitoring of heart rate, blood pressure, respiratory rate, ECG, SpO2 was done every 15min and recorded. Inj. ringer lactate and inj. dextrose-saline 1.5-2 L were infused intraoperatively. The duration of surgery was 2-2.5 h. Inj. ondansetron 4 mg intravenous (IV) was given in immediate postoperative period. Postoperatively all patients were monitored for first 6 h in PACU (Post Anesthesia Care Unit) and thereafter for 24 h in ward. Duration of spinal anesthesia including residual (spinal anesthesiaà effects) was noted in both the groups. Duration of pain relief (analgesia) was calculated as time interval after administration of a study drug till patient feels pain and VAS ≥ 3. At this point rescue analgesia was given in form of inj. diclofenac sodium 75 mg IM. Patients were observed for sedation, nausea, vomiting, respiratory depression, hallucinations, dysphoria, diaphoresis, constipation and urinary retention for 24 h.
Statistical analysis: Sample size of 60 was calculated with assumptions of 95% confidence interval. 13% margin of error and 60% proportion of spinal anesthesia based on previous 2 years record of the institution with 10% dropout rate. Descriptive analysis was carried out in terms of Mean ± SD and percentage. Bivariate analysis was carried out by using unpaired students’ “t” test, Chi-square test and Fischer’s exact test. P value < 0.05 was considered as statistically significant.
RESULTS
60 patients were admitted to the study and none was excluded subsequently. Average duration of spinal anesthesia including residual (spinal anesthesia à its residual effects) was 3.31 h to 3.45 h in both the groups.
Table 1 shows demographic data. Mean age and mean weight of study participants was comparable.
Male: female distribution in both the groups was similar.
Table 2 shows duration of pain relief. Group B had extended duration of analgesia upto > 24 h, while in Group P it was upto 18 h. Although this difference was not statistically significant.
Table 1: Demographic data
Table 2: Duration of pain relief. Data given as n (%)
Yate’s chi square value- 4.7; NS-Not Significant
Table 3 shows time and frequency of rescue analgesia between two groups. Requirement of rescue analgesia was earlier in Group P. Within 18 h 100% of patients required 1st rescue analgesia in Group P, while it was only 76.65% in Group B. Five patients (16. 66%) in Group B did not require 1st rescue analgesic at all within 24 h. 2nd dose of rescue analgesia in 24 h was required in 83. 33% of patients in Group P while it was only 50% in Group B. This difference between two groups was statistically insignificant.
Table 3: Time and frequency of rescue analgesia. Data given as number (%).
Yate’s chi square value 1.1; NS-Not Significant
Table 4: Sedation score
P > 0.05; (NS-Not Significant)
Table 4 shows sedation score. Sedation score was calculated from pre-medication up to 24 h which was statistically not significant between two groups.
Figure 1 shows mean pulse rate per minute. No significant changes in mean pulse rate during intraoperative period and postoperatively up to 24 h.
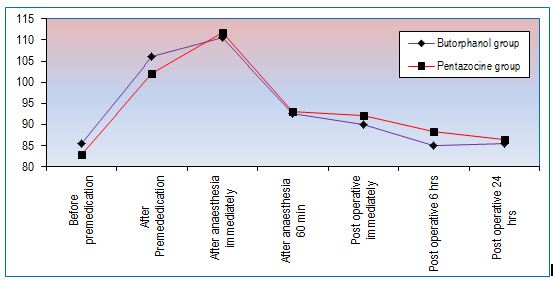
Figure 1: Changes in mean pulse rate per minute
Figure 2 shows mean arterial pressure (MAP) in mmHg, which remains higher in Group P and statistically significant up to 6 h.
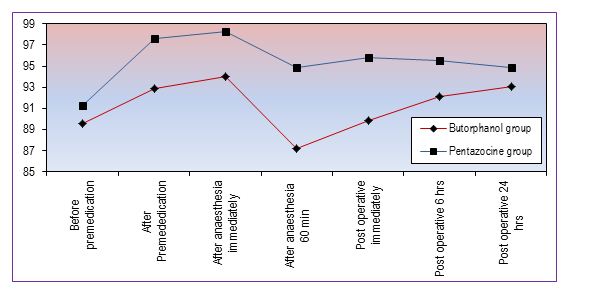
Figure 2: Changes in mean arterial pressure (mmHg)
Nausea was present in 3 (10%) patients of both groups. Vomiting occurred in 2 (6.67%) patients of Group B and 9 (30%) patients of Group P. Dizziness was present in 3 (10%) patients of Group B and 2(6.6%) patients of Group P. Hallucinations, dysphoria, diaphoresis, respiratory depression, constipation or urinary retention were not observed in any patient of either group. Thus no any serious side effect was observed in either group.
DISCUSSION
Our study results along with those from studies elsewhere suggest that butorphanol tartrate, a mixed agonist-antagonist opioid in the dose of 2 mg IM is safe, potent and effective analgesic agent. It is an acceptable alternative to pentazocine lactate as a pre-emptive analgesic due to longer duration of analgesia with greater analgesic efficacy and low incidence of side effects.
Patients develop moderate to severe pain in postoperative period due to tissue injury. The main aim of postoperative pain relief is to provide subjective comfort, inhibiting nociceptive impulses caused by trauma and to blunt autonomic as well as somatic reflexes to pain. This allows the patient to breath, cough and easily ambulant, thus reducing postoperative complications.
Postoperative pain relief can be achieved by many ways, e.g. systemic opioids, intrathecal/epidural local anesthetics with opioids and other analgesics. Patient controlled analgesia (PCA) is an ideal option, but PCA devices are not yet commonly available in all recovery units. Epidural analgesia has become an inseparable part in western countries and resourceful centres in India too. However, it is dependent on an elaborate setup and extra personnel for administration and monitoring, maintaining asepsis, thereby increasing the cost exponentially. The low cost and ease of administration of parenteral route has kept the interest of researchers alive as they are the only effective alternative feasible in low resource settings.
Surgical pain with tissue damage increases responsiveness of both peripheral and central pain pathways. Analgesic interventions are more effective if included the period of noxious stimuli rather than only post injury stage. Preemptive analgesic intervention is initiated before the surgery to attenuate or entirely block central pain sensitization. This reduces pain in postoperative period, rescue analgesic medication and hastens overall recovery.1
Opioids are known potent analgesics used through various routes since many years. But major barriers to access/availability to more recent opioids are shortage of supply due to complicated regulations and problems related to attitude and knowledge regarding pain relief among professionals and public.6 Objective of clinical research in area of pain relief is to identify analgesic agents with atleast the efficacy of pure opioid agonists, but lacking of its addictive and respiratory depressant properties.
Opioid agonist-antagonists e.g. butorphanol, pentazocine and nalbuphine reduce the liability of drug dependence, respiratory depression and other side effects. Butorphanol does not fall under the category of controlled drug. Its use can reduce administrative liability for abuse and lower the number of distribution records associated with schedule II narcotics. It is distributed and stored without special arrangements. Access to recent pure opioid analgesics is limited due to complicated regulations. We have taken pentazocine as a reference substance as it belongs to same group as butorphanol, made easily available by hospital authority and its effects are well documented.
The present study was carried out to compare the preemptive analgesic effect of two opioid drugs. Butorphanol tartrate and pentazocine lactate, both of which have agonist-antagonist property. Present study results showed that inj. butorphanol provided apparently longer duration of analgesia and greater analgesic effect than inj. pentazocine. William C. North and D. R. Tielens 8 studied comparison of butorphanol and pentazocine as postoperative analgesics in general anesthesia, spinal anesthesia and epidural anesthesia. Dose and route of administration of both the drugs were similar to present study. Butorphanol reached its peak effect at 30 min in contrast to 60 min for pentazocine. The duration of effect appeared somewhat longer for butorphanol, though this did not achieve statistical significance, which correlates to our study results. 15% of the patients had dizziness with no significant difference between the treatment groups in the study, which correlates with our study. Butorphanol treatment was significantly better than pentazocine according to many parameters in their study. The onset of analgesia is rapid and analgesic activity is dose related.
In a study by Gilbert MS et al. 9 butorphanol was determined to be 16 times more potent than pentazocine on a weight basis. Both medications provided maximum pain relief within one hour after administration and had comparable duration of action. Drowsiness was the most common side-effect and it appeared to be dose related in all test groups. These findings match with our study results.
Dobkin AB et al.10 compared different doses of butorphanol and pentazocine. Appreciable pain relief developed within 30 min at all dose levels, with a peak analgesic effect apparent at about 1 hour. Satisfactory relief persisted for 4 h in the majority of patients in each group. Vital signs were not appreciably affected, but the patients who received the high dose of analgesics (butorphanol tartrate 4 mg or pentazocine lactate 60 mg) were often quite drowsy. The incidences of other side effects were negligible. The relative potency assay showed that butorphanol was approximately 20 times as potent as pentazocine for up to 4 h. In our study satisfactory pain relief was up to 6 h in both the groups. All these findings match with our study.
When butorphanol was administered epidurally to relieve post cesarean section pain, analgesia of rapid onset was seen with increasing duration and effectiveness observed with increasing dose, approximately 8 h when using 4 mg.11
In the study by Sung YF et al.12 analgesic efficacy of butorphanol was comparable to morphine in balanced anesthesia with less respiratory depression. No hallucinations or dysphoria and minimal nausea and vomiting were similar to present study. Prophylactic injection of ondansetron in the immediate postoperative period was given in present study to prevent nausea and vomiting, which are common side effects of the study drugs.
Peak plasma concentrations of 2.2 ng/ml butorphanol occur between 30-60 min after a single 2 mg IM administration which explains its rapid onset of action. Apparent plasma half-life of butorphanol is between 6 and 10 h. After intramuscular or intravenous administration, butorphanol is widely distributed to tissues with an estimated volume of distribution ranging from 300-900 ml. In humans, after IM injection, the analgesic activity of butorphanol is 15-30 times that of pentazocine.13 Pentazocine has onset in 15-30 min, peak plasma concentration at 60 min and duration of action 2-3 h. It has elimination half-life of 2-3 h. The urinary excretion is rapid as most of the drug is excreted within the first 12 h. Pentazocine efficacy for treatment of severe post-operative pain was somewhat limited because the dose required for good pain relief (40-60 mg) cause excessive side effects. Inter-individual differences in the ability to metabolize pentazocine results in higher concentrations of unchanged drug in some subjects and hence a greater and more intense pharmacological effect. This may account for individual differences in the therapeutic efficacy of the drug and for differences in the incidence of side-effects.12 This might explain duration of analgesia up to 18 h in some patients of Group P and we did not find statistically significant difference between two groups. It has low abuse liability and devoid of properties causing severe respiratory depression, constipation and urine retention.14
Butorphanol and pentazocine produce analgesic effects by primarily stimulating kappa receptors and to some extent sigma opioid receptors. Antagonistic effects at the mu receptors result in a lower potential for respiratory depression.13,14 This explains the stable effect on respiratory system without any depression in present study.
All patients have light to moderate sedation in both groups up to 3 h and light sedation after 3 h up to 24 h. In a study by Philip et al.15 duration of sedation was about 3 h in butorphanol group which matches with our study. Drowsiness was the common side effect observed by other studies also.9,10,16
Observing the effect on hemodynamics, mean pulse rate in patients of Group P was significantly increased at 6 hour because of pain. This result correlates with duration of pain relief, which was less with Group P. After giving rescue analgesic mean pulse rate was settled down and no significant difference between two groups up to 24 h. In the present study significant increase in mean arterial pressure in Group P, that may be due to sympathomimetic effects and rise in plasma epinephrine and norepinephrine level by pentazocine.14,17,18 Butorphanol has no effect on cardiovascular system.13 No effect of Butorphanol on cardiovascular system was observed in present study, which correlates with previous finding by D. A. Laffey and N. H. Kay3 and Mitaka C et al.17
Our study results also matches with the study done by Maryam Tavakoli et al. who observed no changes in blood pressure, pulse rate or respiratory rate with minimal degree and incidence of side effects with butorphanol.19
Indications of butorphanol involve preoperative and pre-induction supplementation, balanced anesthesia and postoperative pain. Butorphanol is a potent analgesic agent; produces limited respiratory depression and an impressive record of safety with a favorable side effect profile. The main advantages of this agent are its low toxicity and very low potential for abuse.20, 21
Limitations of our study are a small sample size and that we included only cases under spinal anesthesia. We have not studied similar drugs for postoperative analgesia after initiation of pain and in high-risk group of patients. These results are not evidence for or against a pre-emptive effect, since such evidence requires a control of the same intervention made at some time point after initiation of the surgical procedure. An ideal pre-emptive or protective analgesic clinical trial should investigate the effect of intense and prolonged multimodal protective interventions versus less aggressive conventional perioperative analgesia on immediate and late postoperative pain as well as on various psychosocial variables.
Strong points of our study are; ease of administration of study drugs, apparently longer duration of analgesia with reduced requirement of rescue medication during postoperative period in butorphanol group compared to pentazocine group. Light sedation with low incidence of other side effects is beneficial during postoperative period.
The present study is helpful in current anesthesia practice in our setting, where pure opioid analgesic availability is limited, but potent totally synthetic opioid analgesics are easily available. Parenteral route of administration is effective and low cost alternative in resource poor settings.
We recommend the use of IM butorphanol as preemptive analgesia as an alternative to pentazocine due to longer duration of effect and greater analgesic efficacy.
CONCLUSION
Our study data along with those from earlier studies suggest that intramuscular use of butorphanol tartrate in the dose of 2 mg is safe and effective analgesic agent. Preemptive use of butorphanol is an alternative to preemptive pentazocine due to longer duration of analgesia with greater analgesic efficacy and low incidence of side effects.
Conflict of interest: None declared by the authors
Authors’ Contribution: HG: Conduction of the study work, literature search, statistical analysis, manuscript preparation.
NP: Conduction of the study work, literature search, data acquisition, statistical analysis.
SG: Concept and design of the study
REFERENCES
1Associate Professor; 3Professor & Head
Dept. of Anesthesiology, GMERS Medical College, Civil Hospital Campus, Sector – 12, Near Pathikasharam, Gandhinagar, Gujarat 382012, (India)
2Associate Professor, Dept. of Anesthesiology, Gujarat Adani Institute of Medical Sciences (GAIMS), G.K.General Hospital Campus,, Bhuj, Gujarat 370001, (India)
Correspondence: Dr Hina Niraj Gadani, 1/T, F/1, Haridham Enclave, B/H Raysan Petrol Pump,
Koba-Gandhinagar Highway, Raysan, Gandhinagar, Gujarat-382007 (India); Mobile: +91-8690501339; E-mail: hinagadani@gmail.com
ABSTRACT
Background: Preemptive analgesia establishes effective antinociception before surgery and continuation of this effective analgesic level well into the postoperative period. Butorphanol tartrate and pentazocine lactate are opioid analgesics with mixed agonist-antagonist properties.
Aim: The aim of the present study was to compare the preemptive analgesic effect of butorphanol and pentazocine given by intramuscular (IM) route as a primary outcome. Secondary outcome was to compare hemodynamic parameters and the side effects profile.
Methodology: A comparative randomized, single blind, and prospective clinical study in sixty patients ASA physical status I and II was carried out. Patients were demographically similar. Patients were randomized to receive either a butorphanol injection (Group B) 2 mg (n=30) or pentazocine injection (Group P) 60 mg (n=30) both IM 60 min before surgery. Lower abdominal surgeries under spinal anesthesia were selected. Duration of pain relief was recorded by visual analogue scale (VAS) postoperatively up to 24 h. Sedation was measured with Cook's sedation score system. Patients were observed for any change in vital signs and any other side effect for 24 h. Rescue analgesia in the form of IM diclofenac sodium 75 mg was given when VAS≥3.
Results: Duration of analgesia was up to 18 h in Group P while it was extended in Group B, but this was statistically not significant. Requirements of rescue analgesia were higher and occurred earlier in Group P, although not statistically significant. Sedation score was also comparable. Hemodynamic changes were not significant with the exception of an increase in mean arterial pressure in Group P. No severe side effects were observed in any patient of either group.
Conclusion: Butorphanol a mixed agonist-antagonist opioid in the dose of 2 mg IM is an acceptable alternative to pentazocine as a pre-emptive analgesic due to longer duration of analgesia and greater analgesic efficacy with low incidence of side effects.
Key words: Injection butorphanol tartrate, injection pentazocine lactate, postoperative analgesia, preemptive analgesia.
Citation: Gadani HN, Patel NB, Gupta SC. Role of butorphanol in preemptive analgesia: A comparison with pentazocine. Anaesth, Pain & Intensive Care 2017;21(1):44-51
Received: 12 Sep 2016; Reviewed: 14 Sep 2016, 6 Jan 2017; Corrected: 16 Sep 2016, 17 Jan 2017; Accepted: 4 Feb 2017
INTRODUCTION
Postoperative pain relief can be achieved by many ways, e. g. parenteral, epidural/local blocks or patient controlled analgesia (PCA); but parenteral method emerges as an effective and low cost alternative in resource poor settings.
Preemptive analgesia is a treatment that is initiated before the surgical procedure in order to reduce the sensitization of peripheral and central pain pathways. Due to this protective effect, it is more effective than a similar analgesic treatment initiated after surgery. Immediate postoperative pain may be reduced and the development of chronic pain may be prevented.1
Pure opioid agonists are effective analgesics but under schedule II narcotics with limited access, more side effects and addictive properties. Synthetic opioid agonist-antagonists share the analgesic property of pure agonists with reduced liability of dependence, respiratory depression and other side effects. Butorphanol, Pentazocine and Nalbuphine are synthetic opioid agonist-antagonists.
Butorphanol tartrate is a synthetic analgesic of 3:14 dihydroxymorphinan structure. It exhibits partial agonist and antagonist activity at the μ-opioid receptor as well as partial agonist activity at the kappa (k)-opioid receptor. Stimulation of these receptors on central nervous system neurons causes an intracellular inhibition of adenylate cyclase, closing of influx membrane calcium channels and opening of membrane potassium channels. This leads to hyperpolarization of the cell membrane and suppression of action potential transmission of ascending pain pathways. When given parenterally it is five times as potent as morphine. k-agonism can cause dysphoria at therapeutic or supertherapeutic doses. This gives butorphanol a lower potential for abuse than other opioid drugs. It is not classified as a controlled drug under the misuse of drugs (1971). 2, 3
Pentazocine is a synthetically-prepared prototypical mixed agonist–antagonist opioid analgesic drug of the benzomorphan class of opioids and is used to treat moderate to severe pain. This compound may exist as one of two enantiomers, named (+)-pentazocine and (−)-pentazocine. (−)-pentazocine is a k-opioid receptor agonist, while (+)-pentazocine is not, instead displaying a ten-fold greater affinity for the σ receptor. Pentazocine was introduced in 1964 for its analgesic properties. Internationally pentazocine is a Schedule III drug under the Convention on Psychotropic Substances. 4
The present clinical study compared pre-emptive analgesic effect and side effects of two mixed agonist-antagonist opioids, butorphanol tartrate and pentazocine lactate given by IM route for lower abdominal surgeries under spinal anesthesia. We choose surgeries under spinal anesthesia, as under a spinal anesthetic, patients typically require significantly lower amounts of narcotics, so they emerge from surgery refreshed and alert rather than groggy and wiped out. Studies have shown a lower rate of post-operative complication with regional anesthesia compared to general anesthesia. 5
Reason for conducting this study is to compare preemptive analgesic efficacy and side effects of potent synthetic opioid agonist-antagonists, butorphanol and pentazocine. Butorphanol does not fall under the category of controlled drug; it is easily distributed and stored without special arrangements. We preferred Pentazocine as a reference substance as it is made easily available by hospital authority and its effects are well documented. Access to other recent pure opioid analgesics is limited due to complicated regulations. 6
METHODOLOGY
Approval for the study was taken from institutional ethical committee. A comparative, randomized, single blind, and prospective clinical study in sixty patients of ASA physical status I and II was carried out at GuruGobind Singh Hospital, Jamnagar, over a period of 1 year. Patients having age of 21-50 years and weighing 50-70 kg were included in the study. Lower abdominal surgeries requiring spinal anesthesia were selected. Patients with any systemic disease, psychological disease, on any chronic medication, history of allergy to any drug and pregnant patients were excluded from the study. All patients were educated about use of VAS which was used for assessment of pain postoperatively. A 0-10 cm line was drawn. Point 0 was considered as no pain and point 10 was considered as worst pain ever. A written informed consent was taken from all patients. Patients were randomly divided into two groups by sealed envelope technique, inj. butorphanol tartrate group (Group B) (n=30) and inj. pentazocine lactate group (Group P) (n=30). Group B patients received inj. butorphanol tartrate 2 mg (1 mg/ml) while Group P patients received inj. pentazocine lactate 60 mg (30 mg/ml) IM 60 min before surgery. The nurse who was giving injection, was not blinded to the study drug. The observer remained blinded to the study drug. Multipara monitor was attached. On arrival in the operation room, initial assessment of degree of sedation was performed using Cook’s sedation score (Box 1).7
| Box 1: Cook’s sedation score.7 | |
| Score | Level |
| 16 – 18 | Not sedated |
| 13 – 15 | Light sedation |
| 8 – 12 | Moderate sedation |
| 5 – 7 | Deep sedation |
Statistical analysis: Sample size of 60 was calculated with assumptions of 95% confidence interval. 13% margin of error and 60% proportion of spinal anesthesia based on previous 2 years record of the institution with 10% dropout rate. Descriptive analysis was carried out in terms of Mean ± SD and percentage. Bivariate analysis was carried out by using unpaired students’ “t” test, Chi-square test and Fischer’s exact test. P value < 0.05 was considered as statistically significant.
RESULTS
60 patients were admitted to the study and none was excluded subsequently. Average duration of spinal anesthesia including residual (spinal anesthesia à its residual effects) was 3.31 h to 3.45 h in both the groups.
Table 1 shows demographic data. Mean age and mean weight of study participants was comparable.
Male: female distribution in both the groups was similar.
Table 2 shows duration of pain relief. Group B had extended duration of analgesia upto > 24 h, while in Group P it was upto 18 h. Although this difference was not statistically significant.
Table 1: Demographic data
| Characteristics | Group B (n=30) |
Group P
(n=30) |
| Age in years (Mean ± SD) | 37.37 ± 8.49 | 35.7 ± 9.74 |
| Weight in kg.(Mean ± SD) | 51.17 ± 6.90 | 53.97 ± 8.24 |
| Sex | ||
| Male | 12(40%) | 14 (46.66%) |
| Female | 18 (60%) | 16 (53.33%) |
Table 2: Duration of pain relief. Data given as n (%)
| Duration of pain relief (h) | Group B (n=30) |
Group P
(n=30) |
p-value | Inference |
| 0-6 | 5 (16.66) | 4 (13.33) | ||
| 6-12 | 14 (46.66) | 21 (70) | ||
| 12-18 | 4 (13.33) | 5 (16.66) | 0.7 | NS |
| 18-24 | 2 (6.66) | 0 (0) | ||
| > 24 | 5 (16.66) | 0 (0) |
Table 3 shows time and frequency of rescue analgesia between two groups. Requirement of rescue analgesia was earlier in Group P. Within 18 h 100% of patients required 1st rescue analgesia in Group P, while it was only 76.65% in Group B. Five patients (16. 66%) in Group B did not require 1st rescue analgesic at all within 24 h. 2nd dose of rescue analgesia in 24 h was required in 83. 33% of patients in Group P while it was only 50% in Group B. This difference between two groups was statistically insignificant.
Table 3: Time and frequency of rescue analgesia. Data given as number (%).
| Time of rescue analgesia in hours (frequency) | Group B (n=30) |
Group P
(n=30) |
p-value | Inference |
| 00-12 (1st) | 19 (63.32) | 25 (83.33) | ||
| 12-18 (1st) | 4 (13.33) | 5 (16.66) | 0.77 | NS |
| 18-24 (1st) | 2 (6.66) | 0 (0) | ||
| 12-24 (2nd) | 15 (50) | 25 (83.33) |
Table 4: Sedation score
| Time at which sedation score taken | Group-B (n=30)
Mean ± SD |
Group-P (n=30)
Mean ± SD |
P value |
| Before premedication | 13 ± 0.00 | 13 ± 0.00 | > 0.05± |
| After 45 min. of premedication | 11.5 ± 0.40 | 11.06 ± 0.00 | > 0.05 |
| After anesthesia | |||
| Immediate | 10.97 ± 0.18 | 11 ± 0.00 | > 0.05 |
| 30 min | 10.97 ± 0.18 | 11 ± 0.00 | 0.05 |
| 60 min | 11 ± 0.28 | 11 ± 0.00 | > 0.05 |
| 90 min | 11.02 ± 0.53 | 11.06 ± 0.25 | > 0.05 |
| 120 min | 11 ± 0.00 | 11.5 ± 0.81 | > 0.05 |
| Post-operative | |||
| Immediate | 11.1 ± 0.48 | 11.2 ± 0.50 | > 0.05 |
| 3 h | 13 ± 0.40 | 12.97 ± 0.18 | > 0.05 |
| 6 h | 13 ± 0.00 | 12.97 ± 0.18 | > 0.05 |
| 12 h | 13 ± 0.00 | 13 ± 0.00 | > 0.05 |
| 24 h | 13 ± 0.00 | 13 ± 0.00 | > 0.05 |
Table 4 shows sedation score. Sedation score was calculated from pre-medication up to 24 h which was statistically not significant between two groups.
Figure 1 shows mean pulse rate per minute. No significant changes in mean pulse rate during intraoperative period and postoperatively up to 24 h.

Figure 1: Changes in mean pulse rate per minute
Figure 2 shows mean arterial pressure (MAP) in mmHg, which remains higher in Group P and statistically significant up to 6 h.

Figure 2: Changes in mean arterial pressure (mmHg)
Nausea was present in 3 (10%) patients of both groups. Vomiting occurred in 2 (6.67%) patients of Group B and 9 (30%) patients of Group P. Dizziness was present in 3 (10%) patients of Group B and 2(6.6%) patients of Group P. Hallucinations, dysphoria, diaphoresis, respiratory depression, constipation or urinary retention were not observed in any patient of either group. Thus no any serious side effect was observed in either group.
DISCUSSION
Our study results along with those from studies elsewhere suggest that butorphanol tartrate, a mixed agonist-antagonist opioid in the dose of 2 mg IM is safe, potent and effective analgesic agent. It is an acceptable alternative to pentazocine lactate as a pre-emptive analgesic due to longer duration of analgesia with greater analgesic efficacy and low incidence of side effects.
Patients develop moderate to severe pain in postoperative period due to tissue injury. The main aim of postoperative pain relief is to provide subjective comfort, inhibiting nociceptive impulses caused by trauma and to blunt autonomic as well as somatic reflexes to pain. This allows the patient to breath, cough and easily ambulant, thus reducing postoperative complications.
Postoperative pain relief can be achieved by many ways, e.g. systemic opioids, intrathecal/epidural local anesthetics with opioids and other analgesics. Patient controlled analgesia (PCA) is an ideal option, but PCA devices are not yet commonly available in all recovery units. Epidural analgesia has become an inseparable part in western countries and resourceful centres in India too. However, it is dependent on an elaborate setup and extra personnel for administration and monitoring, maintaining asepsis, thereby increasing the cost exponentially. The low cost and ease of administration of parenteral route has kept the interest of researchers alive as they are the only effective alternative feasible in low resource settings.
Surgical pain with tissue damage increases responsiveness of both peripheral and central pain pathways. Analgesic interventions are more effective if included the period of noxious stimuli rather than only post injury stage. Preemptive analgesic intervention is initiated before the surgery to attenuate or entirely block central pain sensitization. This reduces pain in postoperative period, rescue analgesic medication and hastens overall recovery.1
Opioids are known potent analgesics used through various routes since many years. But major barriers to access/availability to more recent opioids are shortage of supply due to complicated regulations and problems related to attitude and knowledge regarding pain relief among professionals and public.6 Objective of clinical research in area of pain relief is to identify analgesic agents with atleast the efficacy of pure opioid agonists, but lacking of its addictive and respiratory depressant properties.
Opioid agonist-antagonists e.g. butorphanol, pentazocine and nalbuphine reduce the liability of drug dependence, respiratory depression and other side effects. Butorphanol does not fall under the category of controlled drug. Its use can reduce administrative liability for abuse and lower the number of distribution records associated with schedule II narcotics. It is distributed and stored without special arrangements. Access to recent pure opioid analgesics is limited due to complicated regulations. We have taken pentazocine as a reference substance as it belongs to same group as butorphanol, made easily available by hospital authority and its effects are well documented.
The present study was carried out to compare the preemptive analgesic effect of two opioid drugs. Butorphanol tartrate and pentazocine lactate, both of which have agonist-antagonist property. Present study results showed that inj. butorphanol provided apparently longer duration of analgesia and greater analgesic effect than inj. pentazocine. William C. North and D. R. Tielens 8 studied comparison of butorphanol and pentazocine as postoperative analgesics in general anesthesia, spinal anesthesia and epidural anesthesia. Dose and route of administration of both the drugs were similar to present study. Butorphanol reached its peak effect at 30 min in contrast to 60 min for pentazocine. The duration of effect appeared somewhat longer for butorphanol, though this did not achieve statistical significance, which correlates to our study results. 15% of the patients had dizziness with no significant difference between the treatment groups in the study, which correlates with our study. Butorphanol treatment was significantly better than pentazocine according to many parameters in their study. The onset of analgesia is rapid and analgesic activity is dose related.
In a study by Gilbert MS et al. 9 butorphanol was determined to be 16 times more potent than pentazocine on a weight basis. Both medications provided maximum pain relief within one hour after administration and had comparable duration of action. Drowsiness was the most common side-effect and it appeared to be dose related in all test groups. These findings match with our study results.
Dobkin AB et al.10 compared different doses of butorphanol and pentazocine. Appreciable pain relief developed within 30 min at all dose levels, with a peak analgesic effect apparent at about 1 hour. Satisfactory relief persisted for 4 h in the majority of patients in each group. Vital signs were not appreciably affected, but the patients who received the high dose of analgesics (butorphanol tartrate 4 mg or pentazocine lactate 60 mg) were often quite drowsy. The incidences of other side effects were negligible. The relative potency assay showed that butorphanol was approximately 20 times as potent as pentazocine for up to 4 h. In our study satisfactory pain relief was up to 6 h in both the groups. All these findings match with our study.
When butorphanol was administered epidurally to relieve post cesarean section pain, analgesia of rapid onset was seen with increasing duration and effectiveness observed with increasing dose, approximately 8 h when using 4 mg.11
In the study by Sung YF et al.12 analgesic efficacy of butorphanol was comparable to morphine in balanced anesthesia with less respiratory depression. No hallucinations or dysphoria and minimal nausea and vomiting were similar to present study. Prophylactic injection of ondansetron in the immediate postoperative period was given in present study to prevent nausea and vomiting, which are common side effects of the study drugs.
Peak plasma concentrations of 2.2 ng/ml butorphanol occur between 30-60 min after a single 2 mg IM administration which explains its rapid onset of action. Apparent plasma half-life of butorphanol is between 6 and 10 h. After intramuscular or intravenous administration, butorphanol is widely distributed to tissues with an estimated volume of distribution ranging from 300-900 ml. In humans, after IM injection, the analgesic activity of butorphanol is 15-30 times that of pentazocine.13 Pentazocine has onset in 15-30 min, peak plasma concentration at 60 min and duration of action 2-3 h. It has elimination half-life of 2-3 h. The urinary excretion is rapid as most of the drug is excreted within the first 12 h. Pentazocine efficacy for treatment of severe post-operative pain was somewhat limited because the dose required for good pain relief (40-60 mg) cause excessive side effects. Inter-individual differences in the ability to metabolize pentazocine results in higher concentrations of unchanged drug in some subjects and hence a greater and more intense pharmacological effect. This may account for individual differences in the therapeutic efficacy of the drug and for differences in the incidence of side-effects.12 This might explain duration of analgesia up to 18 h in some patients of Group P and we did not find statistically significant difference between two groups. It has low abuse liability and devoid of properties causing severe respiratory depression, constipation and urine retention.14
Butorphanol and pentazocine produce analgesic effects by primarily stimulating kappa receptors and to some extent sigma opioid receptors. Antagonistic effects at the mu receptors result in a lower potential for respiratory depression.13,14 This explains the stable effect on respiratory system without any depression in present study.
All patients have light to moderate sedation in both groups up to 3 h and light sedation after 3 h up to 24 h. In a study by Philip et al.15 duration of sedation was about 3 h in butorphanol group which matches with our study. Drowsiness was the common side effect observed by other studies also.9,10,16
Observing the effect on hemodynamics, mean pulse rate in patients of Group P was significantly increased at 6 hour because of pain. This result correlates with duration of pain relief, which was less with Group P. After giving rescue analgesic mean pulse rate was settled down and no significant difference between two groups up to 24 h. In the present study significant increase in mean arterial pressure in Group P, that may be due to sympathomimetic effects and rise in plasma epinephrine and norepinephrine level by pentazocine.14,17,18 Butorphanol has no effect on cardiovascular system.13 No effect of Butorphanol on cardiovascular system was observed in present study, which correlates with previous finding by D. A. Laffey and N. H. Kay3 and Mitaka C et al.17
Our study results also matches with the study done by Maryam Tavakoli et al. who observed no changes in blood pressure, pulse rate or respiratory rate with minimal degree and incidence of side effects with butorphanol.19
Indications of butorphanol involve preoperative and pre-induction supplementation, balanced anesthesia and postoperative pain. Butorphanol is a potent analgesic agent; produces limited respiratory depression and an impressive record of safety with a favorable side effect profile. The main advantages of this agent are its low toxicity and very low potential for abuse.20, 21
Limitations of our study are a small sample size and that we included only cases under spinal anesthesia. We have not studied similar drugs for postoperative analgesia after initiation of pain and in high-risk group of patients. These results are not evidence for or against a pre-emptive effect, since such evidence requires a control of the same intervention made at some time point after initiation of the surgical procedure. An ideal pre-emptive or protective analgesic clinical trial should investigate the effect of intense and prolonged multimodal protective interventions versus less aggressive conventional perioperative analgesia on immediate and late postoperative pain as well as on various psychosocial variables.
Strong points of our study are; ease of administration of study drugs, apparently longer duration of analgesia with reduced requirement of rescue medication during postoperative period in butorphanol group compared to pentazocine group. Light sedation with low incidence of other side effects is beneficial during postoperative period.
The present study is helpful in current anesthesia practice in our setting, where pure opioid analgesic availability is limited, but potent totally synthetic opioid analgesics are easily available. Parenteral route of administration is effective and low cost alternative in resource poor settings.
We recommend the use of IM butorphanol as preemptive analgesia as an alternative to pentazocine due to longer duration of effect and greater analgesic efficacy.
CONCLUSION
Our study data along with those from earlier studies suggest that intramuscular use of butorphanol tartrate in the dose of 2 mg is safe and effective analgesic agent. Preemptive use of butorphanol is an alternative to preemptive pentazocine due to longer duration of analgesia with greater analgesic efficacy and low incidence of side effects.
Conflict of interest: None declared by the authors
Authors’ Contribution: HG: Conduction of the study work, literature search, statistical analysis, manuscript preparation.
NP: Conduction of the study work, literature search, data acquisition, statistical analysis.
SG: Concept and design of the study
REFERENCES
- Jørgen B, Dahl, Steen Møiniche. Pre-emptive analgesia. Br Med Bull2004;71(1):13-27. doi: 10. 1093/ bmb/ldh030. [PubMed][Free full text]
- [Online]. [cited 14 September 2016]; Available from: URL:https://en.wikipedia.org/wiki/ Butorphanol
- Laffey DA, Kay NH. Premedication with butorphanol: A comparison with morphine. Br J Anaesth.(1984);56:3637. [PubMed]
- [Online]. [cited 14 September 2016]; Available from: URL:https://en.wikipedia.org/wiki/ Pentazocine.
- Swisher JL. Spinal anesthesia offers many benefits for surgery. SF Gate. [Online]. 2013 Dec 24 [cited 2017 Jan 16]; Available from: URL:http:// sfgate.com/health/article/Spinalanesthesia-offers-many-benefits-forsurgery-5091709.php
- Rajagopal MR, Joranson DE. India: Opioid availability - An update. J Pain Symptom Manage. 2007; 33:615-622. doi:10.1016/j. jpainsymman.2007.02.028. [PubMed][Free full text]
- Melina+Algorithms. Sedation scale of cook and palma. Available from: URL: 8. William C North, D. R. Tielens. Comparison of butorphanol and pentazocine as postoperative analgesics. South Med J.1979 May;72(5):578-580. [PubMed]
- Gilbert MS, Forman RS, Moylan DS, Caruso FS. Butorphanol: a doubleblind comparison with pentazocine in post-operative patients with moderate to severe pain. J Int Med Res. 1976; 4(4):255-64. [PubMed] [Free full text]
- Dobkin AB, Eamkaow S, Caruso FS. Butorphanol and pentazocine in patients with severe postoperative pain. Clin Pharmacol Ther. 1975 Nov; 18(5 Pt 1):547-53. [PubMed]
- Abboud TK, Moore M, Zhu J, Murakawa K, Minehart M, Longhitano M, et al. Epidural Butorphanol or Morphine for the relief of post cesarean section pain: ventilatory responses to carbon dioxide. Anaesth Analg 1987;66:887-93. [PubMed]
- Sung YF, Weinstein MS, Ghani GA. Balanced anaesthesia: A Comparison of butorphanol and morphine. South Med J 1984;77(2):180-182. [PubMed]
- Brogden R.N., Speight T.M., Avery G. S. Pentazocine: a review of its pharmacological properties, therapeutic efficacy and dependence liability. Drugs 1973;5(1):6-91. [PubMed]
- Philip BK, Scott DA, Freiberger D, Gibbs RR, Hunt C, Murray E. Butorphanol compared with fentanyl in general anaesthesia for ambulatory laproscopy. Can J Anaesth 1991 Mar;38(2):183-6. doi: 10. 1007/BF03008141 [PubMed] [Free full text]
- V Trivedi. A comparative clinical study of tramadol v/s pentazocine for sedation and analgesia as premedication (A study of 60 cases). Int J Anesthesiol. 2009;26(1). [Free full text]
- Mitaka C, Sakanishi N, Tsunoda Y, Mishima Y. Comparison of hemodynamic effects of morphine, butorphanol, buprenorphine and pentazocine on ICU patients. Bull Tokyo Med Dent Univ. 1985 Jun;32(2):31-9. [PubMed]
- T Manner, J Kanto, H Scheinin, M Scheinin. Meptazinol and pentazocine: plasma catecholamines and other effects in healthy volunteers. Br J Clin Pharmacol 1987 Dec;24(6):689–697. [PubMed] [Free full text]
- Tavakoli M, Corssen G, Caruso FS. Butorphanol and morphine: A double blind comparison of their parenteral analgesic activity. Anaesth Analg. May 1976;55(3):394-401. [PubMed]
- Rosow CE. Butorphanol in perspective. Acute Care. 1988;12 Suppl 1:2-7. [PubMed]
- Pachter IJ, Evens RP. Butorphanol. Drug Alcohol Depend. 1985 Feb;14(34):325-38. [PubMed]