Prasanna Vadhanan1 Kiruthika Balakrishnan 1
Department of Anesthesiology, Vinayaka Missions Medical College and Hospitals (VMMC), Karaikal 609 609, Pondicherry, (India)
Correspondence: Dr. Prasanna Vadhanan, No 6, P&T Nagar, Mayiladuthurai -609609 (India); Phone: +919486489690; E-mail: vadhanan.prasanna@gmail.com
ABSTRACT:
Background & Objectives: Intrathecal opioids provide an easy and efficient method of prolonging postoperative analgesia due to its action on the spinal opioid receptors. Nalbuphine is a mixed opioid agonist - antagonist which has better side effect profile than morphine. It is easily available in India without a need for narcotics license. The optimal dose of nalbuphine as an adjuvant to intrathecal bupivacaine is not known, as the availability of other narcotics, e.g. fentanyl, sufentanyl etc., in the West has diminished the need to use, and thus to research partial opioids like nalbuphine.
The aim of our study was to compare the duration of postoperative analgesia with 0.8 mg and 1.6 mg of nalbuphine when used as an additive with 0.5% hyperbaric bupivacaine in patients undergoing lower abdominal and lower limb surgeries.
Methodology: 66 patients undergoing various lower abdominal and lower limb surgeries were randomized into 2 groups and received either 0.8 mg or 1.6 mg intrathecal nalbuphine with 3.2 ml of 0.5% hyperbaric bupivacaine. The duration of postoperative analgesia, hemodynamic stability and incidence of adverse effects were noted.
Results: The mean duration of postoperative analgesia in 0.8 mg and 1.6 mg group were 247 ± 12 and 239 ± 10 min respectively (p = 0.007). The incidence of bradycardia was more in 1.6 mg group but did not reach statistical significance. The inability of the higher dose to achieve longer analgesia might be due to a ceiling effect and anti-analgesic actions of nalbuphine.
Conclusion: A dose of 0.8mg of nalbuphine as an intrathecal adjuvant seems to be optimal for providing prolonged postoperative analgesia with minimal side effects.
Key words: Nalbuphine; Postoperative pain; Anesthesia, Spinal; Analgesia
Citation: Comparison of postoperative analgesia with 0.8 mg and 1.6 mg intrathecal nalbuphine; a randomized controlled trial. Anaesth Pain & Intensive Care 2017;21(1):37-43
Received: 25 Sep 2016; Reviewed: 14 Feb 2017; Corrected & Accepted: 11 Mar 2017
INTRODUCTION:
Postoperative pain is associated with lot of negative outcomes like cardiovascular events, poor ventilation, impaired wound healing and poor patient satisfaction. Pain is usually moderate to severe in the immediate postoperative period as the patient recovers from anesthesia. The “First pain” is usually sharp, pricking and localized to the surgical site and is mediated by nociceptors. 1 Central sensitization can occur during periods of exacerbation of acute pain. Adequate analgesia during this period can prevent the negative outcomes and help in early mobilization. Still postoperative pain is undertreated and there is a long way to go towards this objective.2 Intrathecal opioids as an adjuvant to local anesthetics provide an easy and effective way of pain control in the immediate postoperative period. The main reservations against opioids are concerns about side effects like respiratory depression, sedation and availability. Nalbuphine is a semi-synthetic opioid agonist-antagonist analgesic of the phenanthrene series. The drug has been shown to have lesser propensity to cause respiratory depression when compared to morphine in several studies including those in pregnant population.3
The intrathecal dosage of the drug varies from 0.4 to 2.0 mg .4,5 The optimal dose of nalbuphine for this purpose is not clear. The aim of this randomized double blinded controlled clinical study was to compare the duration of postoperative analgesia with two commonly used doses of nalbuphine (0.8 and 1.6 mg) when used as an adjuvant with hyperbaric bupivacaine intrathecally. We hypothesized increasing the dosage from 0.8 to 1.6 mg may not increase the duration of analgesia due to the ceiling effect of partial opioids. The secondary objectives were to observe the hemodynamic parameters and incidence of other side effects.
METHODOLOGY:
A sample size of 60 was determined based on previous studies and alpha and beta error of 5% and 20% (power of 80) respectively and a minimum clinical difference of 30% prolongation of analgesia. Considering for dropouts, a 70 patients of ASA I and II undergoing elective lower abdominal surgeries and lower limb feasible under spinal anesthesia were recruited for the study after obtaining approval from the ethics committee and informed consent from the patients. The study was done in a sub-urban teaching hospital from the month of May to July 2015.
Inclusion criteria were American Society of Anesthesiologist grades 1 & 2, age group 18-60 years, both sexes, BMI 18.5-30, height 145 cm - 180 cm and undergoing elective lower abdominal, pelvic, perineal lower limb surgeries. Exclusion criteria were history of adverse response to nalbuphine or bupivacaine, contraindications to spinal anesthesia, pregnancy, inability to understand visual analogue score (VAS) and chronic drug therapy. Out of 70 patients, 66 fulfilled the eligibility criteria.
Visual analogue scoring was explained to the patients and informed written consent was obtained. All participants were premedicated the night before with oral diazepam and ranitidine and were fasting at least for 6 hours. Selected patients were randomized into two groups - Group A (0.8 ) and Group B (1.6) of 33 each with the help of computer generated numbers from an online random number generator.6 Opaque sealed envelopes are used. After shifting the patients inside the operating room standard monitors (ECG, pulse oximetry, non-invasive blood pressure and nasal capnography) were attached. Patients were preloaded with 500 ml of ringer lactate.
Group A (0.8) patients received 3.2 ml of hyperbaric bupivacaine 0.5% and 0.8 mg of nalbuphine intrathecally in L2-L3 space using a 25G Quincke needle in lateral position. Group B (1.6) patients received the same amount of bupivacaine with 1.6 mg nalbuphine. An insulin syringe was used for precision. The volumes in both syringes were made equal in both groups by adding normal saline. Drugs were prepared by the researcher allotting the groups and handed over to the blinded investigator, who performed the injections and collected the data.
Both the patient and the anesthesiologist administering the drug and monitoring the patient were blinded to the group allotment. Intraoperatively sensory onset (time to achieve loss of sensation to pin prick at T10 level) motor onset (time to achieve a modified Bromage score of 2, Inability to raise leg or flex knees) and maximum level of sensory loss were noted. Hemodynamic parameters (heart rate and blood pressure), oxygen saturation, respiratory rate, level of sedation and nasal Etco2 were monitored and recorded every three minutes intraoperatively. Level of sedation was noted by Campbell scoring system (1-awake, 2-Sedated but arousable, 3-Drowsy, 4-Unarousable).
If patients complained of intraoperative pain, discomfort or reached a VAS score more than 3, general anesthesia was administered and patients were allowed to drop out of the study. Bradycardia (Heart rate less than 60) and hypotension (> 30% fall in mean arterial pressure or < 55mmHg) were treated as per conventions.
Postoperatively the analgesia was assessed by VAS score and time taken for the patient to report a VAS score of 3 was taken as the endpoint. Sensory and motor levels, hemodynamic parameters, sedation levels were also observed. Incidence of nausea, vomiting, pruritus, respiratory depression and any other side effects were recorded.
Statistical analysis was performed using IBM SPSS version 20 and Microsoft Excel 2016 with statistics add-in package installed. Results were expressed as mean ± standard deviation or number (%). Parametric data were compared using Student’s unpaired t test. Anderson-Darling test and Shapiro-Wilk test were done to verify normality of data. Mann Whitney U test was used to compare non parametric data. Chi squared test was used to compare categorical data. Fisher’s exact test was done to determine statistical significance of incidence of side effects. Results were considered significant if p value was equal to or less than 0.05 and highly significant if p < 0.01. Whenever appropriate, the dosage of nalbuphine was the independent variable and the outcome measured was the dependent variable.
RESULT:
Seventy patients were enrolled and 66 fulfilled eligibility criteria. Out of the 66 patients randomized 4 patients from Group 0.8 and 2 patients from group 1.6 dropped out of study (5 patients had prolonged surgery and needed general anesthesia and one patient had a last minute cancellation). They were excluded from the study and an Intention to treat analysis was not done. Statistical analyses were done on 29 patients from 0.8 mg Group and 31 patients from 1.6 mg group.
Consort Flow Diagram
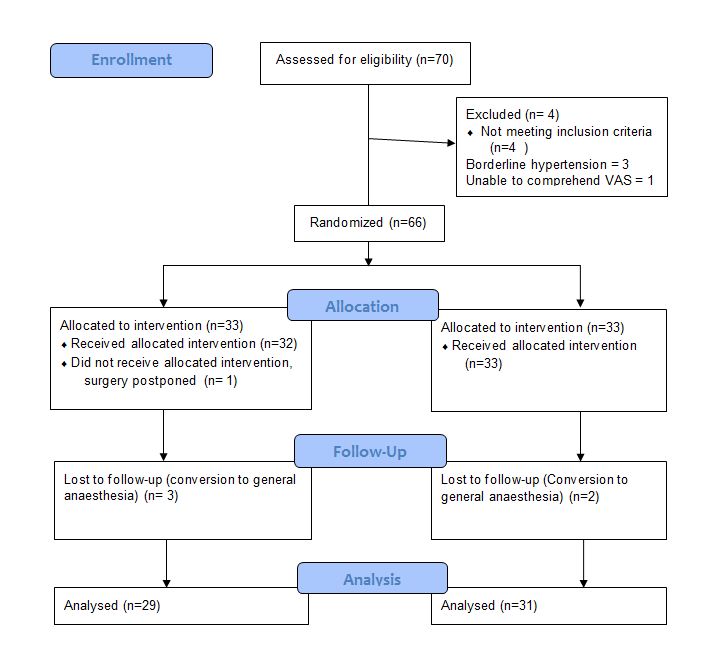
The age, sex, height and weight distributions were similar in both groups. The duration of surgery was highly variable within both groups, ranging from 30 min to 158 min. To check for normality Anderson –Darling and Shapiro Wilk tests were done and the values were found not to be normally distributed (W = 0.921, p = 0.001). Hence Mann Whitney U test was used and showed a p value of 0.246 and hence the duration of surgery did not differ between both groups (Table 1).
Table 1: Demographic data
The types of surgeries were inguinal hernia, abdominal and vaginal hysterectomies, varicose vein stripping etc. (Table 2).
Table 2: Various types of surgeries performed
The mean sensory onset and motor onset time did not differ significantly between both the groups (Table 3). The duration of postoperative analgesia (time to reach VAS of 3) was more in the 0.8 mg group, 247.38 ± 12.2 min vs. 239 ± 10.1 min as compared to the 1.6 mg group (Figure 1).
Table 3: Sensory, motor onset and duration of analgesia
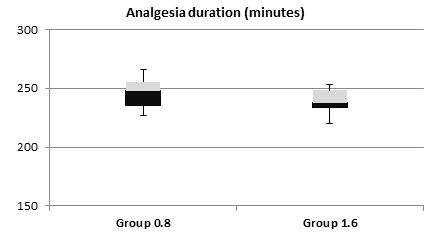
Figure 1: Postoperative analgesia duration
Regarding hemodynamic stability the baseline heart rates were statistically comparable within both groups (p = 0.74); however, intraoperatively the heart rate was persistently lower in 1.6 mg group, with highly significant differences at 30 min and 45 min (p < 0.01) and significant at 150 min, 180 min and 240 min (Figure 2). In fact 5 patients in the 1.6 mg group had symptomatic bradycardia and received injection atropine as compared to one patient in 0.8 mg group. However, the incidence of bradycardia was not statistically significant, (p = 0.196, Fisher’s exact test). Mean arterial pressures were low at 210 min interval (p < 0.01) and 180 min interval (p = 0.04). However, the clinical difference was less than 8 mmHg and none of the patients required intervention (Figure 3). The incidence of other side effects, e.g. nausea and shivering were not statistically significant between the groups (Table 4).
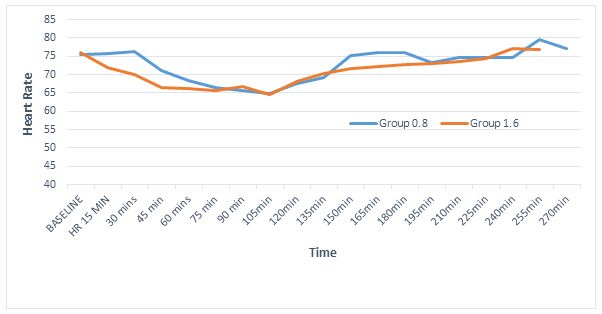
Figure 2: Heart rate changes
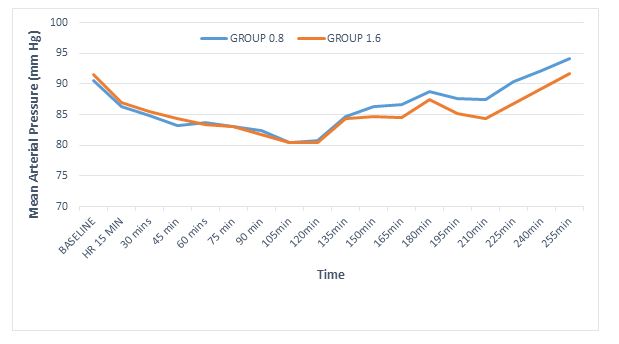
Figure 3: Mean arterial pressure changes
Table 4: Adverse events
Nasal EtCO2 was monitored in all patients and the trend was used to detect respiratory depression. The values were 29.13 ± 2.3 and 29.16 ± 2.6 in 0.8 mg and 1.6 mg groups respectively. The values in both groups did not alter significantly from the baseline. Similarly respiratory rate and oxygen saturation changes were minimal and not significant.
A post hoc power analysis was done. With Cohen’s effect size of 0.78, 2 sided t test using the mean and standard deviations of the primary outcome, a value of 0.84 was obtained which was acceptable.
DISCUSSION:
Intrathecal nalbuphine had been found to be equally effective to morphine regarding postoperative analgesia and a superior side effect profile. Various doses have been used and a progressively increasing duration of postoperative analgesia has been suggested up to doses of 0.8. mg.7 On the other hand the incidence of adverse effects increases with higher doses. Our study has compared a ‘conventional’ dose of 0.8 mg and a ‘high’ dose of 1.6 mg as an adjuvant to 0.5% hyperbaric bupivacaine with respect to postoperative analgesia.
The primary outcome, duration of postoperative analgesia was approximately 4 hours in both the groups. The results correlate with studies by Gomaa H et al.8 who reported analgesia duration of 231.83 ± 15.73 min with 0.8 mg nalbuphine and Tiwari et al.9 who reported a duration of 278.5 ± 6.04 with 0.4 mg nalbuphine.
Inability of higher doses of nalbuphine to prolong analgesia demonstrate a ceiling effect, where in higher doses cannot achieve more analgesia, but can increase the incidence of adverse effects. Interestingly nalbuphine can have an anti-analgesic effect in higher doses, which might partly explain inability of the high dose to achieve more analgesia. It has been recently suggested by Gear R et al., that “Nalbuphine-induced activation of caudate (perhaps by disinhibition, as opioids are inhibitory) initiates pain-enhancing connectivity with other regions, and, although the receptor(s) involved is not known, and blockade of this connectivity by naloxone abolishes this pronociceptive effect”.10
The anti-analgesic effect is pronounced in males. A 5 mg dose of nalbuphine caused more postoperative pain than placebo in male patients.11 This might be due to sex related due to sexual dimorphism of kappa opioid receptors12 upon which nalbuphine exerts its predominant action.
The sensory and motor onset did not differ significantly between both groups. Several studies have monitored 2 segmental regression times intraoperatively. We feel checking for segmental levels intraoperatively in abdominal surgeries and in a calm, sedated patient is cumbersome and impractical.
Heart rate was significantly lower in 1.6 mg group at several instances. Bradycardia is a known side effect of nalbuphine. 5/31 patients of 1.6 mg group had bradycardia and 1/31 had nausea compared 1/29 and nil in 0.8 mg group respectively. Even though the difference mean heart rates were significant, the incidence of bradycardia was not statistically significant. However, significance cannot be attributed to this factor as the study is not powered to detect adverse events.
Nasal EtCo2 monitoring’s accuracy depends upon the design of the cannula.13 Zhang et al. reported a significant correlation between nasal PetCO2 and PaCo2. The correlation coefficients had approximate values, 0.832 (P < 0.0001) for PaCO2 with PetCO2 through the nose and 0.836 (P < 0.0001) for PaCO2 with PetCO2 through the pharynx.14 We have monitored nasal PetCO2 to detect respiratory depression due to nalbuphine and the values were not significantly raised from baseline.
LIMITATIONS:
Conversion to general anesthesia was required in 5 patients and an intention to treat analysis was not done. Various strategies have been suggested to deal with missing data and each has its limitations. The resultant unequal sample size might make the treatment effect difficult to interpret. Visual analogue scale, the primary measurement tool is a subjective scale and can vary between patients with same degree of painful stimuli, however it is a validated tool and widely used to assess pain. Two segmental regression time was not calculated as we find it unpleasant to test sensory levels in the middle of abdominal surgeries, though several studies mention it.
CONCLUSION:
Intrathecal nalbuphine in doses of 0.8 mg and 1.6 mg as an adjuvant to 0.5% hyperbaric bupivacaine prolongs the duration of postoperative analgesia by approximately 4 hours, which can be highly valuable in preventing acute postoperative pain and its adverse effects. A dose of 0.8 mg provides better postoperative analgesia with less adverse events than a 1.6 mg dose.
Conflict of interest: Nil declared by the authors
Authors’ Contribution: PV - Concept, study designing, manuscript preparation, statistical analysis design. KB - Conduction, data collection..
REFERENCES:
Department of Anesthesiology, Vinayaka Missions Medical College and Hospitals (VMMC), Karaikal 609 609, Pondicherry, (India)
Correspondence: Dr. Prasanna Vadhanan, No 6, P&T Nagar, Mayiladuthurai -609609 (India); Phone: +919486489690; E-mail: vadhanan.prasanna@gmail.com
ABSTRACT:
Background & Objectives: Intrathecal opioids provide an easy and efficient method of prolonging postoperative analgesia due to its action on the spinal opioid receptors. Nalbuphine is a mixed opioid agonist - antagonist which has better side effect profile than morphine. It is easily available in India without a need for narcotics license. The optimal dose of nalbuphine as an adjuvant to intrathecal bupivacaine is not known, as the availability of other narcotics, e.g. fentanyl, sufentanyl etc., in the West has diminished the need to use, and thus to research partial opioids like nalbuphine.
The aim of our study was to compare the duration of postoperative analgesia with 0.8 mg and 1.6 mg of nalbuphine when used as an additive with 0.5% hyperbaric bupivacaine in patients undergoing lower abdominal and lower limb surgeries.
Methodology: 66 patients undergoing various lower abdominal and lower limb surgeries were randomized into 2 groups and received either 0.8 mg or 1.6 mg intrathecal nalbuphine with 3.2 ml of 0.5% hyperbaric bupivacaine. The duration of postoperative analgesia, hemodynamic stability and incidence of adverse effects were noted.
Results: The mean duration of postoperative analgesia in 0.8 mg and 1.6 mg group were 247 ± 12 and 239 ± 10 min respectively (p = 0.007). The incidence of bradycardia was more in 1.6 mg group but did not reach statistical significance. The inability of the higher dose to achieve longer analgesia might be due to a ceiling effect and anti-analgesic actions of nalbuphine.
Conclusion: A dose of 0.8mg of nalbuphine as an intrathecal adjuvant seems to be optimal for providing prolonged postoperative analgesia with minimal side effects.
Key words: Nalbuphine; Postoperative pain; Anesthesia, Spinal; Analgesia
Citation: Comparison of postoperative analgesia with 0.8 mg and 1.6 mg intrathecal nalbuphine; a randomized controlled trial. Anaesth Pain & Intensive Care 2017;21(1):37-43
Received: 25 Sep 2016; Reviewed: 14 Feb 2017; Corrected & Accepted: 11 Mar 2017
INTRODUCTION:
Postoperative pain is associated with lot of negative outcomes like cardiovascular events, poor ventilation, impaired wound healing and poor patient satisfaction. Pain is usually moderate to severe in the immediate postoperative period as the patient recovers from anesthesia. The “First pain” is usually sharp, pricking and localized to the surgical site and is mediated by nociceptors. 1 Central sensitization can occur during periods of exacerbation of acute pain. Adequate analgesia during this period can prevent the negative outcomes and help in early mobilization. Still postoperative pain is undertreated and there is a long way to go towards this objective.2 Intrathecal opioids as an adjuvant to local anesthetics provide an easy and effective way of pain control in the immediate postoperative period. The main reservations against opioids are concerns about side effects like respiratory depression, sedation and availability. Nalbuphine is a semi-synthetic opioid agonist-antagonist analgesic of the phenanthrene series. The drug has been shown to have lesser propensity to cause respiratory depression when compared to morphine in several studies including those in pregnant population.3
The intrathecal dosage of the drug varies from 0.4 to 2.0 mg .4,5 The optimal dose of nalbuphine for this purpose is not clear. The aim of this randomized double blinded controlled clinical study was to compare the duration of postoperative analgesia with two commonly used doses of nalbuphine (0.8 and 1.6 mg) when used as an adjuvant with hyperbaric bupivacaine intrathecally. We hypothesized increasing the dosage from 0.8 to 1.6 mg may not increase the duration of analgesia due to the ceiling effect of partial opioids. The secondary objectives were to observe the hemodynamic parameters and incidence of other side effects.
METHODOLOGY:
A sample size of 60 was determined based on previous studies and alpha and beta error of 5% and 20% (power of 80) respectively and a minimum clinical difference of 30% prolongation of analgesia. Considering for dropouts, a 70 patients of ASA I and II undergoing elective lower abdominal surgeries and lower limb feasible under spinal anesthesia were recruited for the study after obtaining approval from the ethics committee and informed consent from the patients. The study was done in a sub-urban teaching hospital from the month of May to July 2015.
Inclusion criteria were American Society of Anesthesiologist grades 1 & 2, age group 18-60 years, both sexes, BMI 18.5-30, height 145 cm - 180 cm and undergoing elective lower abdominal, pelvic, perineal lower limb surgeries. Exclusion criteria were history of adverse response to nalbuphine or bupivacaine, contraindications to spinal anesthesia, pregnancy, inability to understand visual analogue score (VAS) and chronic drug therapy. Out of 70 patients, 66 fulfilled the eligibility criteria.
Visual analogue scoring was explained to the patients and informed written consent was obtained. All participants were premedicated the night before with oral diazepam and ranitidine and were fasting at least for 6 hours. Selected patients were randomized into two groups - Group A (0.8 ) and Group B (1.6) of 33 each with the help of computer generated numbers from an online random number generator.6 Opaque sealed envelopes are used. After shifting the patients inside the operating room standard monitors (ECG, pulse oximetry, non-invasive blood pressure and nasal capnography) were attached. Patients were preloaded with 500 ml of ringer lactate.
Group A (0.8) patients received 3.2 ml of hyperbaric bupivacaine 0.5% and 0.8 mg of nalbuphine intrathecally in L2-L3 space using a 25G Quincke needle in lateral position. Group B (1.6) patients received the same amount of bupivacaine with 1.6 mg nalbuphine. An insulin syringe was used for precision. The volumes in both syringes were made equal in both groups by adding normal saline. Drugs were prepared by the researcher allotting the groups and handed over to the blinded investigator, who performed the injections and collected the data.
Both the patient and the anesthesiologist administering the drug and monitoring the patient were blinded to the group allotment. Intraoperatively sensory onset (time to achieve loss of sensation to pin prick at T10 level) motor onset (time to achieve a modified Bromage score of 2, Inability to raise leg or flex knees) and maximum level of sensory loss were noted. Hemodynamic parameters (heart rate and blood pressure), oxygen saturation, respiratory rate, level of sedation and nasal Etco2 were monitored and recorded every three minutes intraoperatively. Level of sedation was noted by Campbell scoring system (1-awake, 2-Sedated but arousable, 3-Drowsy, 4-Unarousable).
If patients complained of intraoperative pain, discomfort or reached a VAS score more than 3, general anesthesia was administered and patients were allowed to drop out of the study. Bradycardia (Heart rate less than 60) and hypotension (> 30% fall in mean arterial pressure or < 55mmHg) were treated as per conventions.
Postoperatively the analgesia was assessed by VAS score and time taken for the patient to report a VAS score of 3 was taken as the endpoint. Sensory and motor levels, hemodynamic parameters, sedation levels were also observed. Incidence of nausea, vomiting, pruritus, respiratory depression and any other side effects were recorded.
Statistical analysis was performed using IBM SPSS version 20 and Microsoft Excel 2016 with statistics add-in package installed. Results were expressed as mean ± standard deviation or number (%). Parametric data were compared using Student’s unpaired t test. Anderson-Darling test and Shapiro-Wilk test were done to verify normality of data. Mann Whitney U test was used to compare non parametric data. Chi squared test was used to compare categorical data. Fisher’s exact test was done to determine statistical significance of incidence of side effects. Results were considered significant if p value was equal to or less than 0.05 and highly significant if p < 0.01. Whenever appropriate, the dosage of nalbuphine was the independent variable and the outcome measured was the dependent variable.
RESULT:
Seventy patients were enrolled and 66 fulfilled eligibility criteria. Out of the 66 patients randomized 4 patients from Group 0.8 and 2 patients from group 1.6 dropped out of study (5 patients had prolonged surgery and needed general anesthesia and one patient had a last minute cancellation). They were excluded from the study and an Intention to treat analysis was not done. Statistical analyses were done on 29 patients from 0.8 mg Group and 31 patients from 1.6 mg group.
Consort Flow Diagram

The age, sex, height and weight distributions were similar in both groups. The duration of surgery was highly variable within both groups, ranging from 30 min to 158 min. To check for normality Anderson –Darling and Shapiro Wilk tests were done and the values were found not to be normally distributed (W = 0.921, p = 0.001). Hence Mann Whitney U test was used and showed a p value of 0.246 and hence the duration of surgery did not differ between both groups (Table 1).
Table 1: Demographic data
| Parameter | Group 0.8 | Group 1.6 | p - value |
| Age (years) | 41.5 ± 10.2 | 42.9 ± 8 | 0.579 (students t) |
| Height (Cm) | 163.44 ± 7.08 | 163.87 ± 7.71 | 0.827 |
| Weight (Kg) | 59.3 ± 8.49 | 58.2 ± 6.47 | 0.57 |
| Sex (M:F) | 13:16 | 13:18 | 0.821 (Chi squared) |
| Duration of surgery (min) |
97.03 ± 41 (Range 35-160) |
84.74 ± 37.9 (range 30-158) |
0.246 (Mann Whitney) |
The types of surgeries were inguinal hernia, abdominal and vaginal hysterectomies, varicose vein stripping etc. (Table 2).
Table 2: Various types of surgeries performed
| Procedure | Group 0.8 | Group 1.6 | Total |
| Appendectomy Abdominal hysterectomy Vaginal hysterectomy Uterine myomectomy Hydrocele eversion Gluteal abscess I&D Multiple lipoma excision Hernioplasty Varicose vein stripping Pilonidal sinus excision Skin grafting |
3 6 3 1 2 0 1 7 3 1 2 |
3 4 3 2 5 1 0 6 2 2 3 |
6 10 6 3 7 1 1 13 5 3 5 |
| Total | 29 | 31 | 60 |
Table 3: Sensory, motor onset and duration of analgesia
| Parameter | Group 0.8 | Group 1.6 | p value (Unpaired t) |
| Sensory onset (min) | 2.57 ± 0.23 | 2.62 ± 0.28 | 0.429 |
| Motor onset (min) | 2.79 ± 0.234 | 2.85 ± 0.24 | 0.379 |
| Duration of analgesia (min) | 247.38 ± 12.2 | 239.23 ± 10.1 | 0.007* (Significant) |

Figure 1: Postoperative analgesia duration
Regarding hemodynamic stability the baseline heart rates were statistically comparable within both groups (p = 0.74); however, intraoperatively the heart rate was persistently lower in 1.6 mg group, with highly significant differences at 30 min and 45 min (p < 0.01) and significant at 150 min, 180 min and 240 min (Figure 2). In fact 5 patients in the 1.6 mg group had symptomatic bradycardia and received injection atropine as compared to one patient in 0.8 mg group. However, the incidence of bradycardia was not statistically significant, (p = 0.196, Fisher’s exact test). Mean arterial pressures were low at 210 min interval (p < 0.01) and 180 min interval (p = 0.04). However, the clinical difference was less than 8 mmHg and none of the patients required intervention (Figure 3). The incidence of other side effects, e.g. nausea and shivering were not statistically significant between the groups (Table 4).

Figure 2: Heart rate changes

Figure 3: Mean arterial pressure changes
Table 4: Adverse events
| Adverse event | Group 0.8
(n=29) |
Group 1.6
(n=31) |
p value
(Fisher’s exact test) |
| Bradycardia | 1 | 5 | 0.196 |
| Nausea | 0 | 2 | 0.492 |
| Shivering | 0 | 3 | 0.238 |
A post hoc power analysis was done. With Cohen’s effect size of 0.78, 2 sided t test using the mean and standard deviations of the primary outcome, a value of 0.84 was obtained which was acceptable.
DISCUSSION:
Intrathecal nalbuphine had been found to be equally effective to morphine regarding postoperative analgesia and a superior side effect profile. Various doses have been used and a progressively increasing duration of postoperative analgesia has been suggested up to doses of 0.8. mg.7 On the other hand the incidence of adverse effects increases with higher doses. Our study has compared a ‘conventional’ dose of 0.8 mg and a ‘high’ dose of 1.6 mg as an adjuvant to 0.5% hyperbaric bupivacaine with respect to postoperative analgesia.
The primary outcome, duration of postoperative analgesia was approximately 4 hours in both the groups. The results correlate with studies by Gomaa H et al.8 who reported analgesia duration of 231.83 ± 15.73 min with 0.8 mg nalbuphine and Tiwari et al.9 who reported a duration of 278.5 ± 6.04 with 0.4 mg nalbuphine.
Inability of higher doses of nalbuphine to prolong analgesia demonstrate a ceiling effect, where in higher doses cannot achieve more analgesia, but can increase the incidence of adverse effects. Interestingly nalbuphine can have an anti-analgesic effect in higher doses, which might partly explain inability of the high dose to achieve more analgesia. It has been recently suggested by Gear R et al., that “Nalbuphine-induced activation of caudate (perhaps by disinhibition, as opioids are inhibitory) initiates pain-enhancing connectivity with other regions, and, although the receptor(s) involved is not known, and blockade of this connectivity by naloxone abolishes this pronociceptive effect”.10
The anti-analgesic effect is pronounced in males. A 5 mg dose of nalbuphine caused more postoperative pain than placebo in male patients.11 This might be due to sex related due to sexual dimorphism of kappa opioid receptors12 upon which nalbuphine exerts its predominant action.
The sensory and motor onset did not differ significantly between both groups. Several studies have monitored 2 segmental regression times intraoperatively. We feel checking for segmental levels intraoperatively in abdominal surgeries and in a calm, sedated patient is cumbersome and impractical.
Heart rate was significantly lower in 1.6 mg group at several instances. Bradycardia is a known side effect of nalbuphine. 5/31 patients of 1.6 mg group had bradycardia and 1/31 had nausea compared 1/29 and nil in 0.8 mg group respectively. Even though the difference mean heart rates were significant, the incidence of bradycardia was not statistically significant. However, significance cannot be attributed to this factor as the study is not powered to detect adverse events.
Nasal EtCo2 monitoring’s accuracy depends upon the design of the cannula.13 Zhang et al. reported a significant correlation between nasal PetCO2 and PaCo2. The correlation coefficients had approximate values, 0.832 (P < 0.0001) for PaCO2 with PetCO2 through the nose and 0.836 (P < 0.0001) for PaCO2 with PetCO2 through the pharynx.14 We have monitored nasal PetCO2 to detect respiratory depression due to nalbuphine and the values were not significantly raised from baseline.
LIMITATIONS:
Conversion to general anesthesia was required in 5 patients and an intention to treat analysis was not done. Various strategies have been suggested to deal with missing data and each has its limitations. The resultant unequal sample size might make the treatment effect difficult to interpret. Visual analogue scale, the primary measurement tool is a subjective scale and can vary between patients with same degree of painful stimuli, however it is a validated tool and widely used to assess pain. Two segmental regression time was not calculated as we find it unpleasant to test sensory levels in the middle of abdominal surgeries, though several studies mention it.
CONCLUSION:
Intrathecal nalbuphine in doses of 0.8 mg and 1.6 mg as an adjuvant to 0.5% hyperbaric bupivacaine prolongs the duration of postoperative analgesia by approximately 4 hours, which can be highly valuable in preventing acute postoperative pain and its adverse effects. A dose of 0.8 mg provides better postoperative analgesia with less adverse events than a 1.6 mg dose.
Conflict of interest: Nil declared by the authors
Authors’ Contribution: PV - Concept, study designing, manuscript preparation, statistical analysis design. KB - Conduction, data collection..
REFERENCES:
- Gupta A, Kaur K, Sharma S, Goyal S, Arora S, Murthy RS. Clinical aspects of acute post-operative pain management & its assessment. J Adv Pharm Technol Res. 2010 Apr;1(2):97-108. [PubMed] [Free full text]
- Breivik H, Stubhaug A. Management of acute postoperative pain: still a long way to go! 2008 Jul 15;137(2):233-4.doi: 10.1016/j.pain.2008.04.014. [PubMed]
- Zeng Z, Lu J, Shu C, Chen Y, Guo T, Wu QP. A Comparision of Nalbuphine with Morphine for Analgesic Effects and Safety : Meta-Analysis of Randomized Controlled Trials Sci Rep. 2015; 5: 10927. doi: 10.1038/srep10927. [PubMed] [Free full text]
- Jyothi B, Gowda S, Shaikh SI, et al. A comparison of analgesic effect of different doses of intrathecal nalbuphine hydrochloride with bupivacaine and bupivacaine alone for lower abdominal and orthopedic surgeries. Indian J Pain 2014;28:18-23. [Free full text]
- Gupta K, Rastogi B, Gupta PK et al. Intrathecal nalbuphine versus intrathecal fentanyl as adjuvant to 0.5% hyperbaric bupivacaine for orthopedic surgery of lower limbs under subarachnoid block: A comparative evaluation. Indian J Pain. 2016;30(2):90-95. [Free full text]
- RESEARCH RANDOMIZER. [Online]. [cited 20th May 2015] Available from: URL: http://www.randomizer.org
- Mukherjee A, Pal A, Agrawal J, Mehrotra A, Dawar N. Intrathecal nalbuphine as an adjuvant to subarachnoid block: What is the most effective dose? Anesth Essays Res. 2011 Jul-Dec; 5(2): 171–175. doi: 10.4103/0259-1162.94759. [PubMed] [Free full text]
- Gomaa HH, Mohamed NN, Zoheir HAH, Ali MS. A comparison between post-operative analgesia after intrathecal nalbuphine with bupivacaine and intrathecal fentanyl with bupivacaine after cesarean section. Egypian J Anaesth. 2014; 30(4):405-410. [Free full text]
- Tiwari AK, Tomar GS, Agrawal J. Intrathecal bupivacaine in comparision with a combination of nalbuphine and bupivacaine for subarachnoid block: A randomized prospective double blind clinical study. Am J Ther 2013;20:592-5. doi: 10.1097/MJT.0b013e31822048db. [PubMed]
- Gear R, Becerra L, Upadhyay J, Bishop J, Wallin D, Pendse G, et al. Pain facilitation brain regions activated by nalbuphine are revealed by pharmacological fMRI. PLoS One. 2013; 8(1): e50169. doi: 10.1371/journal.pone.0050169. [PubMed] [Free full text]
- Gear RW, Gordon NC, Hossaini-Zadeh M, Lee JS, Miaskowski C, Paul SM, et al. A subanalgesic dose of morphine eliminates Nalbuphine anti-analgesia in postoperative pain. J Pain. 2008;9(4):337-41. doi: 10.1016/j.jpain.2007.11.011. [PubMed] [Free full text]
- Rasakham K, Liu-Chen LY. Sex differences in kappa opioid pharmacology. Life Sci. 2011 Jan 3; 88(0): 10.1016/j.lfs.2010.10.007. doi: 10.1016/j.lfs.2010.10.007. [PubMed] [Free full text]
- Ebert TJ, Novalija J, Uhrich TD, Barney JA. The effectiveness of oxygen delivery and reliability of carbon dioxide waveforms: a crossover comparison of 4 nasal cannulae. Anesth Analg. 2015 Feb;120(2):342-8. doi: 10.1213/ANE.0000000000000537. [PubMed]
- Zhang C, Wang M, Wang R, Wang W. Accuracy of end-tidal CO2 measurement through the nose and pharynx in nonintubated patients during digital subtraction cerebral angiography. J Neurosurg Anesthesiol. 2013 Apr;25(2):191-6. doi: 10.1097/ANA.0b013e31827c9d5a. [PubMed]