Marwa Ahmed El-Oraby 1*, Aliaa El-Said Shaban 2 , Ahmed Ali El-Dada 3 , Abd El-Aziz Hamed El-Badawy 3
Authors’ affiliations:
Abstract
Background: Sepsis-induced myocardial dysfunction (SIMD) occurs in 50% of septic patients and is characterized by reduced ejection fraction (EF), cardiac index, impaired contractility, and diastolic dysfunction (DD). In sepsis-induced cardiomyopathy (SICM), EF shows initial significant deterioration on the 1st day, then final improvement at the end of the study. This study evaluated the value of different parameters measured with trans-thoracic echocardiography (TTE) in the diagnosis and prognosis of SIMD in the surgical intensive care unit (SICU).
Methodology: This prospective cohort study was conducted on 100 patients, aged from 18 to 50 years admitted to SICU being affected by sepsis or septic shock. TTE parameters [EF, tricuspid annular systolic excursion (TAPSE), inferior vena cava (IVC) diameter, E/A ratio and grading of DD and hemodynamic parameters [mean arterial blood pressure (MAP), heart rate (HR), central venous pressure (CVP)] on admission, three day post-admission and after one week.
Results: The mortality rate was 45%. DD was found in 90%. The mortality group had higher DD, higher HR, and lower MAP than the surviving group, with an insignificant difference in LVEF, TAPSE, IVC, and CVP on the 3rd and 7th days. Sepsis-induced cardiomyopathy (SICM) was found in 31% of surviving patients. DD (grade III had the highest mortality followed by grade I then grade II), HR >110 bpm, and MAP < 65mmHg are independent factors that negatively affect the duration of survival significantly.
Conclusion: TTE in patients with sepsis or septic shock is vital for diagnosis and prognosis. DD, tachycardia (HR >110 bpm), and hypotension (MAP < 65mmHg) are independent predictors of mortality in those patients. Patients with SICM (little reversible impairment of LV systolic function) had a good prognosis.
Keywords: Sepsis Induced Myocardial Dysfunction, Diastolic dysfunction, Sepsis, Septic Shock
Preregistration: The study was registered in the Ethical Committee of Faculty of Medicine, Tanta University, Tanta, Egypt (approval number: 31728/08/17)
Abbreviations: SIMD–Sepsis-induced myocardial dysfunction; SICM–sepsis-induced cardiomyopathy; TTE– transthoracic echocardiogram. EF–Ejection fraction; DD–diastolic dysfunction; MD–Myocardial dysfunction; TAPSE–tricuspid annular systolic excursion; SICU–surgical intensive care unit
Citation: El-Oraby MA, Shaban AES, El-Dada AA, El-Badawy AEH. Echocardiographic evaluation of sepsis induced myocardial dysfunction in patients with sepsis or septic shock: a prospective cohort study. Anaesth pain intensive care 2021;25(2):150-162.
DOI: 10.35975/apic.v25i2.1463
Received: 6 November 2020, Reviewed: 30 December 2020, Accepted: 3 February 2021
Introduction
Sepsis and septic shock are major healthcare problems affecting millions of people around the world each year. Sepsis is now defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Septic shock is a subset of sepsis with circulatory and cellular/metabolic dysfunction associated with a higher risk of mortality. Myocardial dysfunction (MD) is a common finding in septic patients, and approximately 50% of septic patients exhibit signs of MD.1
The heart is the only part of the circulatory system that constantly responds to changes in peripheral hemodynamics, and it is difficult to differentiate between the direct effect of sepsis on the heart (myocardium) and the cardiac responses to alterations in preload, afterload, and neuro-humoral activity occurring in sepsis. MD resulting from sepsis is characterized by reduced ejection fraction (EF) and cardiac index, impaired contractility, and diastolic dysfunction (DD). MD is an essential part of multi-organ failure that is triggered mainly by sepsis.2
Sepsis-induced cardiomyopathy (SICM) is a complication associated with sepsis and septic shock, which was first identified by Parker in 1984. SIMD is a reversible myocardial depression in sepsis and septic shock patients and has three main characteristics; left ventricular (LV) dilatation, reduced EF, and recovery within 7–10 days.3
Transthoracic echocardiography (TTE) is a common bedside reliable noninvasive examination, and it has made it easy to evaluate the hemodynamics of SIMD, either systolic or diastolic LV dysfunction and right ventricular (RV) dysfunction. Moreover, new echocardiographic methods such as speckle-tracking echocardiography represent an exciting method in the early diagnosis of SIMD in septic shock.4
This study evaluated the value of different parameters measured with TTE in SIMD’s diagnosis and prognosis in the surgical intensive care unit (SICU).
Methodology
This prospective study was conducted on 100 patients, aged from 18 to 50 y admitted to SICU being affected by sepsis or septic shock from October 2017 to October 2019 in Tanta University Hospitals. Written informed consent was obtained from each patient, either by the patient or they were next of kin. The study was approved by institutional ethical committees.
Patients were included in our study only after the fulfillment of the following criteria:
After the stabilization of the airway, breathing, standard continuous monitoring of electrocardiogram (ECG), respiratory rate and arterial blood pressure, and central venous catheter insertion was done. Fluid resuscitation was done, empirical antibiotics and routine investigations as C- reactive protein (CRP), total leukocyte count (TLC), serum lactate, blood culture, liver function, and renal function were ordered.
Hemodynamic measurements [MAP, heart rate (HR) and central venous pressure (CVP)] were recorded on admission, three days post-admission and one-week post-admission.
TTE examinations
All patients underwent TTE examination on admission, three days post-admission and one week post-admission. The examination started by positioning the patient in the supine position or preferred in the left lateral decubitus position. Then, the following TTE parameters were measured in the included patients:
LV ejection fraction (LVEF) as a surrogate to LV systolic function:
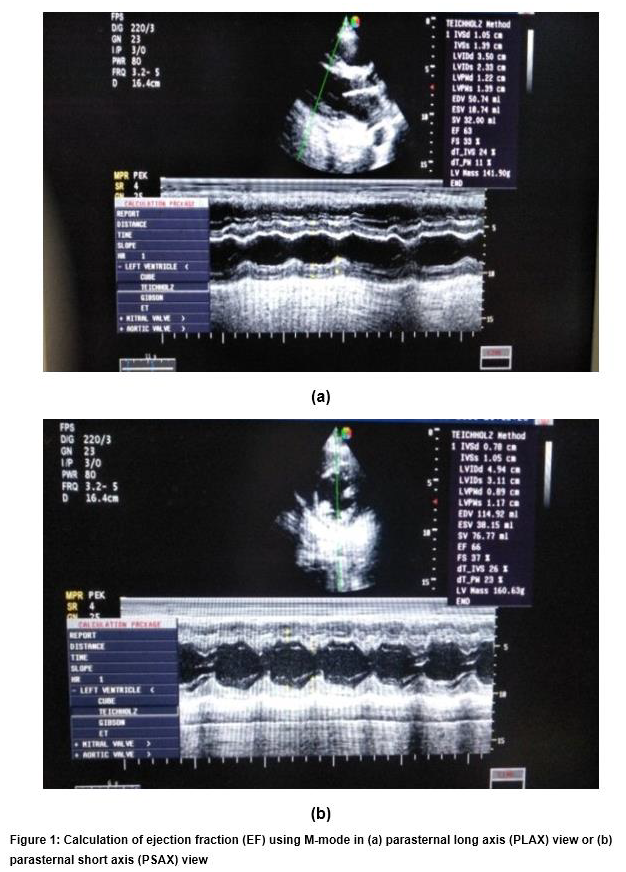
LVEF was measured using a standard M-Mode technique in the parasternal long axis (PLAX) view or parasternal short axis (PSAX) view (Figure 1). Then, we calculated LVEF by measurement of both left ventricular end-systolic diameter (LVESD) and end-diastolic diameter (LVEDD). The normal value of EF is 55%–75%. LV systolic dysfunction (SD) was classified as mild dysfunction (40% < LVEF < 50%), moderate dysfunction (20% < LVEF < 40%) and severe dysfunction (LVEF < 20%).
LV diastolic function
LV diastolic filling patterns were evaluated by the mitral inflow pulsed-wave Doppler examination in the apical four-chamber view. The pulsed wave Doppler recording a sample volume of 1 to 3 mm should be located at the open MV leaflets' tips. This sample volume should be positioned toward the lateral wall since blood flows naturally through the mitral valve in this direction; both peak velocities E (early diastole) and A (atrial contraction) were measured. These peak velocities of the early (E) and late (A) filling waves were derived from the mitral inflow velocity curve, and the ratio of early to late peak velocities (E/A) was calculated (Figure 2).

In normal diastolic physiology, there are two phases. The first one occurs primarily during the early phase of diastole blood flow into the LV. The first phase leads to a peak mitral inflow velocity during early diastole called (E-phase). It is higher than the peak mitral inflow velocity of the 2nd phase combined with atrial contraction, which is called (A-phase) and E/A ratio of 1–2).
DD Grade (I): mild DD demonstrate abnormal relaxation but without elevated end-diastolic filling pressure (E velocity less than A velocity and decreased of early to late ventricular filling velocity E/A ratio to <1).
DD Grade (II): moderate or “pseudo-normal” DD demonstrate abnormal relaxation with increased LV end-diastolic filling pressure and increase the left atrial pressure so (E velocity is greater than A and E/A ratio of 1–2).
DD Grade (III): severe DD demonstrates a further progressive decline in LV compliance (significantly increased LV stiffness) with restrictive filling and atrial contraction is inadequate to force blood into the non-compliant LV (E velocity much higher than A and E/A ratio more than 2). We can differentiate normal diastolic function from DD grade II by using either Valsalva maneuver or by using tissue Doppler imaging, which measures E`wave, which represents the movement of mitral valve annulus if E`< 8 cm means impaired relaxation (DD grade II).
Right ventricle (RV) function: (Figure 3)

RV function was assessed by measuring tricuspid annular systolic excursion (TAPSE) measured in the apical four-chamber image using M-mode. The precursor should be as possible as be aligned along the free wall of RV and perpendicular to the lateral tricuspid annulus, so the precursor becomes parallel to the motion of the tricuspid valve annulus. The tricuspid annulus maximum excursion indicates the distance moved by the leading edge of the tricuspid annulus to the apex measured and expressed in centimeters (from the end of diastole to the end of systole). Normal TAPSE was ≥ 1.6 cm, and impaired TAPSE was < 1.6 cm.
Inferior vena cava (IVC) diameter
IVC was measured using the M-mode imaging in the subcostal view window, and the M-mode precursor is placed roughly 1.0 to 2.0 cm away from the right atrium through the IVC (Figure 4).

IVC diameter was classified as normal (1.5–2.5 cm), collapsed if diameter < 1.5 cm and dilated IVC > 2.5 cm.
SICM patients were defined as a group of the surviving patients who had EF less than 50% and decrease ≥ 10% of the EF compared to the baseline, which recovered within 1–2 weeks. If the baseline measurement appeared uncertain, we assessed EF on admission and defined baseline EF. The concept of recovery based on EF improvement to baseline or > 10% relative to the EF’s initial admission evaluation.3,5
Statistical analysis
Statistical analysis of data was performed by SPSS v26 (IBM©, Chicago, IL, USA). The Kolmogorov–Smirnov test checked the normality of data, and all variables were normally distributed. Quantitative variables were presented as mean and standard deviation (SD) and were compared using the paired t-test. Qualitative variables were presented as frequency and percentage (%) and were analyzed using the chi-square test. Kaplan–Meier survival analysis was used to compare survival between groups. Logistic regression analysis was used to show the independent predictors. The level of significance was adopted at p < 0.05.
Results
Patients' age ranged from 18 to 50 years with a mean value being 38.29 ± 8.71 y. There were 48 (48%) males and 52 (52%) females.
EF was significantly decreased on the 3rd day compared to 1st day (55.64 ± 6.23% vs. 58.24 ± 7.58% respectively (p ˂ 0.001*) then increased again to 60.13 ± 5.57% (p = 0.275). Impaired EF was found in 20 (20%) patients on the 1st day, and only 4 (7%) from 55 surviving patients on the 7th day (significantly decreased compared with 1st day, p = 0.036) (Table 1).

SICM was found in 17 patients from 55 survived patients (Table 2).
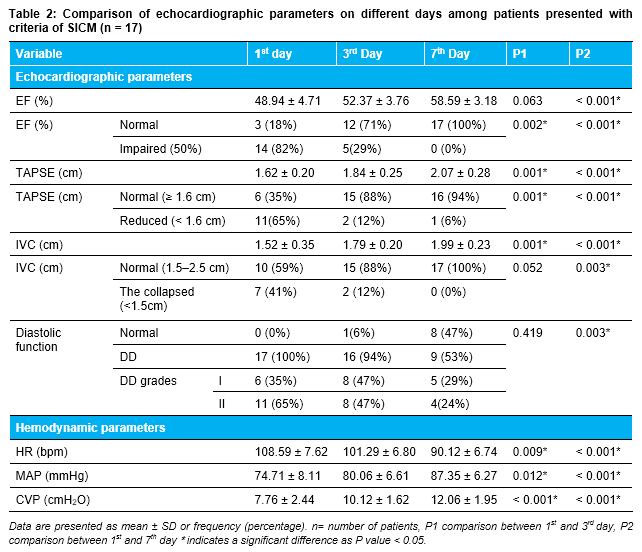
Data are presented as mean ± SD or frequency (percentage). n= number of patients, EF: ejection fraction, TAPSE: tricuspid annular systolic excursion, IVC: inferior vena cava, DD: Diastolic dysfunction, HR: heart rate, bpm: beats per minute, MAP: mean arterial blood pressure, CVP: central venous pressure. P1: Comparison between 1st and 3rd day, P2: Comparison between 1st and 7th day *indicates a significant difference as p < 0.05.
Forty-five patients (45%) passed away on the 7th day; 22 patients (22%) died by the 3rd day, and 23 patients (23%) died between the 4th to 7th day.
The comparison between the surviving group and mortality groups on the 7th day showed no significant difference in EF, TAPSE, IVC, and CVP on 1st day. DD on 1st day was significantly higher in the mortality group than in the surviving group (47.7% vs 20%, P = 0.004). HR was significantly higher, and MAP was significantly lower in the mortality group compared to the surviving group (Table 3).

Concerning DD grading, we found that all patients (100%) with DD grade III died, shown the worst prognosis. The mortality percentage related to DD grade I counted as 53.2%, and the least mortality was 32.4%, related to grade II (Table 3).
Regarding patients with impaired systolic function (EF < 50%), there was no significant difference in the mortality group compared with the surviving group (25% vs. 13%, respectively, p = 0.321), indicating that systolic function cannot be used as a predictor of mortality in septic patients (Table 2).
Kaplan–Meier survival analysis
The hazard ratio of mortality was significantly higher 3.06 times (95% CI: 1.26–7.41) in patients with DD compared with patients with normal diastolic function. The hazard ratio of mortality was insignificantly higher [1.59 times (95% CI: 0.76–3.32)] in patients with low EF compared with patients with normal EF. The hazard ratio of mortality was insignificantly lower [0.86 times (95% CI: 0.42–1.77)] in patients with reduced TAPSE compared to patients with normal TAPSE. The hazard ratio of mortality was insignificantly higher [1.38 times (95% CI: 0.71–2.68)] in patients with collapsed IVC compared to patients with normal IVC. The hazard ratio of mortality was significantly higher [3.72 times (95% CI: 1.99–6.94)] in patients with HR > 110 bpm compared to patients with HR ≤ 110 bpm (Figure 5e). The hazard ratio of mortality was significantly higher [9.24 times (95%CI: 3.86–22.11)] in patients with MAP < 65 mmHg compared to patients with MAP ≥ 65 mmHg.
Multiple Logistic Regression Models
In our study, the most independent variables that affect mortality were DD, tachycardia (HR > 110 bpm), and hypotension (MAP < 65 mmHg), which significantly affected the duration of survival negatively (p = 0.0497*, < 0.001, and < 0.001, respectively) (Table 4).

Discussion
Sepsis can lead to reduced LV systolic function, hyperdynamic LV function, and DD. Recognition of these types of MD with echocardiography could result in better outcomes.6 MD, caused by sepsis, is a complicated process because of many factors, including host response to infection, dynamic cardiovascular system (CVS) adaptation to the disease, and resuscitation effects. This entity’s pathophysiology is multifactorial; and extracellular, cellular and systemic mechanisms have been suggested, such as coronary blood flow maldistribution, injury of the myocardium, complementary (C5a) contractile myocyte failure, neutrophil activation by cytokines (TNF, IL-1β, IL-6), Ca manipulation dysregulation, and dysfunction of mitochondria that cause cellular hypoxia.7
Our results revealed that LV systolic function (LVEF), RV function (TAPSE), IVC diameter, and CVP have no role in predicting mortality.
In agreement with our study, Rolando et al.8 studied 53 septic patients, 26% of them had SD, and 23% of them had both SD and DD. They have shown that SD was not a predictor of mortality and uncorrelated with an increased death rate, as compared to DD, which was 84% of patients.
Moreover, Ng et al.9 reported the finding of a crucial study on SIMD comparing septic shock patients (41.9% mortality) with a control group of patients with sepsis (0% mortality), using speckle-tracking echocardiography to identify MD in septic patients. They assessed SD using global longitudinal strain (GLS) and found a higher degree of MD in patients with the septic shock group compared to the control group. On the other side, LVEF was similar in both groups (59% in the study group versus 61% in the control group, p = 0.169).
This finding was in agreement with a meta-analysis recorded by Sanfilippo10 on 581 septic patients. They reported that 29.6% of them had SD, and, there is no correlation between SD and mortality, while 48% of them had DD indicates a significant correlation between DD and mortality.
In contrast to our study, Patil et al. found that the mean LVEF was 35.70% and more than 70% of septic patients had SD, and the mortality rate was high in patients with impaired EF (< 50%) more than patients with normal EF (15% vs. 34%, respectively).11
Another study by Vieillard-Baron and colleagues on 67 patients free from previous cardiac disease and survived for > 48 h stated that global LV hypokinesia (EF < 45%) was found in 60% of the septic patients.12
The left atrium (LA) size is used as a diagnostic criterion for DD. However, due to adaptation to chronically high LV filling pressures, LA enlargement developed slowly. In sepsis and septic shock, LA enlargement does not appear quickly, and diastolic abnormalities are commonly acute. So, in this particular condition, LA size is unlikely to be used as an accurate indicator of diastolic filling abnormalities. Therefore, we chose to use the Doppler evaluation of LV function and filling in a similar situation.12
In our study, the prevalence of DD was 90%, and it was a strong independent predictor of mortality in patients with sepsis and septic shock.
Our results agreed with Sturgess et al.,13 in a retrospective study of 94 general ICU patients who underwent TTE, demonstrated that DD occurred in 67%.
Also, Brown et al.,14 in one of the largest prospective studies assessing 78 septic patients admitted to ICU and underwent TTE within 6 h of admission, after 18 to 32 h for evaluating LVDD, they observed that 36.5% of patients had DD on the 1st ECHO, while 61.8% had DD on at least one ECHO and total mortality was 16.5%. Also, they observed that the largest mortality occurred among patients with grade I DD (37.5%), concluding that DD was a strong independent predictor of mortality in those septic patients.
This agreed with Dantas and Costa,15 as they observed a high alarming occurrence (84%) of DD in septic patients using the E/e’ ratio, which is one of the tissue Doppler imaging (TDI) measurements that used to identify DD, also observed that DD was the only echocardiographic parameter separately correlated with mortality even after age and disease severity adjustment.
The potential influence of DD on the occurrence of mortality of septic patients in ICU can be linked to various factors: First, abnormal relaxation leads to an early decrease of LV filling in addition to an increased proportion of LA contraction to late LV filling and a gradual increase in LV filling pressure. Second, reported tachycardia and hypovolemia with sepsis result in more reduction of LV early loading by shortening the time of diastole and consequently decreasing CO. The significant increase in LV filling pressures presented with DD that may encourage a positive fluid balance, which may exacerbate non-cardiogenic sepsis-related pulmonary edema or may even result in the right cardiac failure. This is indicated by the increased (RVEDA/LVEDA) ratio in ICU dead patients without the association of acute cor-pulmonale.16
From an opposite perspective, there are some studies investigating septic patients in contrast to our results, and these studies revealed that DD has fewer roles in predicting mortality as Bouhemad et al.17 recorded the incidence of LV DD alone 20% in 54 post-operative patients with septic shock using transesophageal echocardiography (TEE).
Also, Gonzalez et al.,18 in their largest study on 540 patients in the ICU with septic shock over 5 years reported that ICU mortality was 35%, and DD was found in 31% of patients. The incidence of DD appeared to be more in the non-survivor group than in the survived group (36% versus 28%, respectively). So, the study concluded that DD could be correlated with ICU mortality, but more studies are needed to declare or prove the prognostic role of DD in septic shock.
Interestingly, in the current study, we found that LV diastolic function was the most significant ECHO parameter. Among patients with normal diastolic function, the mortality rate was 20% (2 patients only from 10 patients presented by normal diastolic function), while the mortality rate was 47.7% among patients with DD, and the worst mortality rate was DD grade III 100% (all patients n=6) followed by DD grade I mortality (53.2%) among its patients (25 patients from 47), then less incidence of mortality was observed in patients with DD grade II (32.4%).
Hemodynamic parameters have an important role in our study. We found some vital data have a pivotal role in predicting mortality in ICU patients, while others do not have any role.
In our study, CVP, as a surrogate for patient volume status, may help in resuscitation with no aid in prognosis or clinical course of septic patients. In contrast to CVP, we found that HR and MAP were strong predictors of mortality in septic patients. Increased HR and decreased MAP in dead patients compared to survived patients reflect that the worst vital data, the worst prognosis, and more increased mortality.
Weng et al.,19 upon following up sepsis and septic shock patients in ICU concluded that HR was significantly lower in the surviving group 103 bpm versus 120 bpm in the non-survived groups. So, HR is a reliable indicator of mortality, while MAP and CVP have no prognostic value in predicting 90-day mortality.
In disagreement with our study Etchecopar-Chevreuil et al.20 in their study, reported that HR and MAP have no value in expecting mortality in ICU septic patients, as MAP was (114 mmHg in the surviving group versus 124 mmHg in the mortality group), also mean HR was (84 bpm in the survived group versus 82 bpm in the mortality group).
The follow-up of 55 survived patients up to the 7th day, we noticed that there was a significant improvement in hemodynamic and echo parameters, compared with the 1st day. When analyzed, these patients were divided into two groups: patients with criteria of SICM (N=17), and others without criteria of SICM (N=38) patients. One of the main finding of this study was precluding a group of patients had SICM criteria (17 patients 31% from survived population and 17% from all study population), in which EF showed initial significant deterioration on the 1st day, then the final significant improvement at the end of the study, as mean LVEF was (48.94 ± 4.71), (52.37 ± 3.76) and (58.59 ± 3.18), on the 1st, 3rd and 7th days respectively (p < 0.001) and patients presented with normal EF ≥ 50% were 3 (18%), 12 (71%) and 17 (100%) patients on the 1st, 3rd and 7th days respectively and patients with impaired EF < 50% were 14 (82%), 5 (29%) patients on the 1st and 3rd days and no patient presented with impaired EF on the 7th day of study.
Other echo parameters showed significant progressive improvement, as mean TAPSE was (1.62 ± 0.20), (1.84 ± 0.25) and (2.07 ± 0.28) cm and mean IVC was (1.52 ± 0.35), (1.79 ± 0.20) and (1.99 ± 0.23) cm on the 1st, 3rd and 7th days, respectively. On the 7th day, the SICM patients’ group was with more improved diastolic function as follows: on the 1st day, there was no patient presented with normal diastolic function, while 17 patients (100%) presented with DD, as (6 patients (35%) with DD grade I and 11 patients (65%) with DD grade II). On the 3rd day, there was only 1 patient (6%) with normal diastolic function, while 16 patients (94%) with DD, as (8 patients (47%) with DD grade I and 8 patients (47%) with DD grade II). On the 7th day, there were 8 patients (47%) with normal diastolic function, while 9 patients (53%) with DD, as (5 patients (29%) with DD grade I and 4 patients (24%) with DD grade II). There was a statistically significant improvement between the 1st and 7th days regarding diastolic function as (p-value 0.003).
Consequently, those patients were diagnosed as SICM patients, facing little reversible impairment of LV systolic function, and they were the groups with a good prognosis.
In agreement with our study Parker and colleagues, after their seminal, works on 20 patients with diagnosed septic shock, as 10 patients suffered from moderate to severe impairment of their EF with values ˂ 40% and 13 of 20 patients survived their episode. Survivors had initial EF with a mean of 32%, and serial scans of the survivor revealed a gradual recovery to normal EF and ventricular volume by 10 days after the beginning of shock. In contrast, non-survivors had normal initial EF and ventricular volumes that did not alter during serial studies.21
The prevalence rate of SICM, recorded in previous research, was 18%-65%22,23 while Sato et al.,5 in their largest study on 210 septic patients demonstrated that SICM patients were 13.8%.
On the other side, Ng et al.9 stated that EF does not appear suitable for assessing MD in septic patients, and no association between EF and mortality. However, caution should be exercised about the analysis of the finding on MD reversibility.
More interestingly, De Geer et al.24 observed no change in systolic function on performing three serial ECHO assessments (on the 1st, 3rd, 4th days, and after ICU discharge) and did not find significant reversibility in GLS. Consequently, MD reversibility questions remain unanswered and need further studies to be explained.
The reliability of the reversibility of SICM depends on the optimum timing of the revaluation echocardiographic examination, which needs to be described. Laboratory studies have documented that lipopolysaccharides in sepsis can produce cardiac fibrosis, which may induce long-term influence on myocardial contractility. Sanfilippo et al.4 selected the time of weaning off vasopressors as an early recovery measurable parameter, during ICU stay of patients. However, to fully understand the extent of myocardial impairment and recovery, follow-up using TTE assessment may be more effectively performed until patients’ full recovery and discharge from ICU.
Although all previous studies that have reported MD in septic shock, most of them could not justify why survivors displayed more marked MD. Levy et al. showed myocardial hibernation in sepsis using positron emission tomography (PET), magnetic resonance imaging (MRI), and single-photon emission tomography. Myocardial hibernation (is an adaptive response to ischemia and hypoxia) is the strongest method to maintain cardiac myocytes by down-regulation of energy requirements and oxygen consumption. It is an adaptive reaction to preserve myocardial viability to prevent the activation of the cell-death pathway and to help full recovery in the future. Also, postmortem examination of septic patients demonstrated a histologic absence of injury and little cell death, although severe organ dysfunction, but more serial study is needed to confirm this hypothesis.25
Septic patients always presented with tachycardia, which worsens LV filling time (decrease diastolic time), and both increased HR and DD are strong predictors of mortality, so many studies were needed to evaluate the benefits of using β-blocker in septic patients for increasing diastolic time, improve LV filling and cardiac performance and its effect on mortality.
Limitations
This study is limited by relatively small sample size, being a single-center study, and a short duration of follow-up (7 days). Also, we did not evaluate the efficacy of therapeutic changes that directly resulted from the initial TTE.
Sepsis-induced myocardial dysfunction; SICM–sepsis-induced cardiomyopathy; TTE– transthoracic echocardiogram
Conclusions
Identification of sepsis-induced myocardial dysfunction using transthoracic echocardiogram in patients with sepsis or septic shock is essential and helpful in the prediction of mortality. Diastolic dysfunction grade III had the highest mortality followed by grade I, grade II), HR >110 bpm, and MAP < 65 mmHg are independent predictors of mortality in those patients. Patients with sepsis-induced cardiomyopathy (little reversible impairment of LV systolic function) had a good prognosis.
Authors’ contributions
All authors contributed to the paper.
Conflict of interest
None declared by the authors.
References
Authors’ affiliations:
- Assistant lecturer, Department of Anesthesiology, Surgical Intensive Care and Pain Medicine, Faculty of Medicine, Tanta University, Tanta, Egypt.
- Lecturer of Cardiology, Faculty of Medicine, Tanta University, Tanta, Egypt.
- Professor of Anesthesiology, Surgical Intensive Care and Pain Medicine, Faculty of Medicine, Tanta University, Tanta, Egypt.
Abstract
Background: Sepsis-induced myocardial dysfunction (SIMD) occurs in 50% of septic patients and is characterized by reduced ejection fraction (EF), cardiac index, impaired contractility, and diastolic dysfunction (DD). In sepsis-induced cardiomyopathy (SICM), EF shows initial significant deterioration on the 1st day, then final improvement at the end of the study. This study evaluated the value of different parameters measured with trans-thoracic echocardiography (TTE) in the diagnosis and prognosis of SIMD in the surgical intensive care unit (SICU).
Methodology: This prospective cohort study was conducted on 100 patients, aged from 18 to 50 years admitted to SICU being affected by sepsis or septic shock. TTE parameters [EF, tricuspid annular systolic excursion (TAPSE), inferior vena cava (IVC) diameter, E/A ratio and grading of DD and hemodynamic parameters [mean arterial blood pressure (MAP), heart rate (HR), central venous pressure (CVP)] on admission, three day post-admission and after one week.
Results: The mortality rate was 45%. DD was found in 90%. The mortality group had higher DD, higher HR, and lower MAP than the surviving group, with an insignificant difference in LVEF, TAPSE, IVC, and CVP on the 3rd and 7th days. Sepsis-induced cardiomyopathy (SICM) was found in 31% of surviving patients. DD (grade III had the highest mortality followed by grade I then grade II), HR >110 bpm, and MAP < 65mmHg are independent factors that negatively affect the duration of survival significantly.
Conclusion: TTE in patients with sepsis or septic shock is vital for diagnosis and prognosis. DD, tachycardia (HR >110 bpm), and hypotension (MAP < 65mmHg) are independent predictors of mortality in those patients. Patients with SICM (little reversible impairment of LV systolic function) had a good prognosis.
Keywords: Sepsis Induced Myocardial Dysfunction, Diastolic dysfunction, Sepsis, Septic Shock
Preregistration: The study was registered in the Ethical Committee of Faculty of Medicine, Tanta University, Tanta, Egypt (approval number: 31728/08/17)
Abbreviations: SIMD–Sepsis-induced myocardial dysfunction; SICM–sepsis-induced cardiomyopathy; TTE– transthoracic echocardiogram. EF–Ejection fraction; DD–diastolic dysfunction; MD–Myocardial dysfunction; TAPSE–tricuspid annular systolic excursion; SICU–surgical intensive care unit
Citation: El-Oraby MA, Shaban AES, El-Dada AA, El-Badawy AEH. Echocardiographic evaluation of sepsis induced myocardial dysfunction in patients with sepsis or septic shock: a prospective cohort study. Anaesth pain intensive care 2021;25(2):150-162.
DOI: 10.35975/apic.v25i2.1463
Received: 6 November 2020, Reviewed: 30 December 2020, Accepted: 3 February 2021
Introduction
Sepsis and septic shock are major healthcare problems affecting millions of people around the world each year. Sepsis is now defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Septic shock is a subset of sepsis with circulatory and cellular/metabolic dysfunction associated with a higher risk of mortality. Myocardial dysfunction (MD) is a common finding in septic patients, and approximately 50% of septic patients exhibit signs of MD.1
The heart is the only part of the circulatory system that constantly responds to changes in peripheral hemodynamics, and it is difficult to differentiate between the direct effect of sepsis on the heart (myocardium) and the cardiac responses to alterations in preload, afterload, and neuro-humoral activity occurring in sepsis. MD resulting from sepsis is characterized by reduced ejection fraction (EF) and cardiac index, impaired contractility, and diastolic dysfunction (DD). MD is an essential part of multi-organ failure that is triggered mainly by sepsis.2
Sepsis-induced cardiomyopathy (SICM) is a complication associated with sepsis and septic shock, which was first identified by Parker in 1984. SIMD is a reversible myocardial depression in sepsis and septic shock patients and has three main characteristics; left ventricular (LV) dilatation, reduced EF, and recovery within 7–10 days.3
Transthoracic echocardiography (TTE) is a common bedside reliable noninvasive examination, and it has made it easy to evaluate the hemodynamics of SIMD, either systolic or diastolic LV dysfunction and right ventricular (RV) dysfunction. Moreover, new echocardiographic methods such as speckle-tracking echocardiography represent an exciting method in the early diagnosis of SIMD in septic shock.4
This study evaluated the value of different parameters measured with TTE in SIMD’s diagnosis and prognosis in the surgical intensive care unit (SICU).
Methodology
This prospective study was conducted on 100 patients, aged from 18 to 50 y admitted to SICU being affected by sepsis or septic shock from October 2017 to October 2019 in Tanta University Hospitals. Written informed consent was obtained from each patient, either by the patient or they were next of kin. The study was approved by institutional ethical committees.
Patients were included in our study only after the fulfillment of the following criteria:
- Clinical criteria of sepsis, including suspected infection by quick sepsis-related organ failure assessment (qSOFA) score ≥ 2, including alteration in mental status, systolic blood pressure < 100 mmHg, and respiratory rate > 22/min
The dysfunction of organs can be confirmed by an acute change in the sepsis-related organ failure assessment (SOFA) variables ≥ 2 points subsequent to the infection, which includes (PaO2/FiO2 ratio < 300, Glasgow Coma Scale score < 15, mean arterial blood pressure (MAP) < 70 mmHg, serum creatinine >1.2 mg/dl or urine output < 0.5 ml/kg, serum bilirubin > 1.2 mg/dl and platelet count < 150 X 103 /µl) - Clinical criteria of septic shock are persistent hypotension needing vasopressors to maintain MAP > 65 mmHg and having a serum lactate level 2 mmol/L, although adequate volume resuscitation.

After the stabilization of the airway, breathing, standard continuous monitoring of electrocardiogram (ECG), respiratory rate and arterial blood pressure, and central venous catheter insertion was done. Fluid resuscitation was done, empirical antibiotics and routine investigations as C- reactive protein (CRP), total leukocyte count (TLC), serum lactate, blood culture, liver function, and renal function were ordered.
Hemodynamic measurements [MAP, heart rate (HR) and central venous pressure (CVP)] were recorded on admission, three days post-admission and one-week post-admission.
TTE examinations
All patients underwent TTE examination on admission, three days post-admission and one week post-admission. The examination started by positioning the patient in the supine position or preferred in the left lateral decubitus position. Then, the following TTE parameters were measured in the included patients:
LV ejection fraction (LVEF) as a surrogate to LV systolic function:
LVEF was measured using a standard M-Mode technique in the parasternal long axis (PLAX) view or parasternal short axis (PSAX) view (Figure 1). Then, we calculated LVEF by measurement of both left ventricular end-systolic diameter (LVESD) and end-diastolic diameter (LVEDD). The normal value of EF is 55%–75%. LV systolic dysfunction (SD) was classified as mild dysfunction (40% < LVEF < 50%), moderate dysfunction (20% < LVEF < 40%) and severe dysfunction (LVEF < 20%).
LV diastolic function
LV diastolic filling patterns were evaluated by the mitral inflow pulsed-wave Doppler examination in the apical four-chamber view. The pulsed wave Doppler recording a sample volume of 1 to 3 mm should be located at the open MV leaflets' tips. This sample volume should be positioned toward the lateral wall since blood flows naturally through the mitral valve in this direction; both peak velocities E (early diastole) and A (atrial contraction) were measured. These peak velocities of the early (E) and late (A) filling waves were derived from the mitral inflow velocity curve, and the ratio of early to late peak velocities (E/A) was calculated (Figure 2).

In normal diastolic physiology, there are two phases. The first one occurs primarily during the early phase of diastole blood flow into the LV. The first phase leads to a peak mitral inflow velocity during early diastole called (E-phase). It is higher than the peak mitral inflow velocity of the 2nd phase combined with atrial contraction, which is called (A-phase) and E/A ratio of 1–2).
DD Grade (I): mild DD demonstrate abnormal relaxation but without elevated end-diastolic filling pressure (E velocity less than A velocity and decreased of early to late ventricular filling velocity E/A ratio to <1).
DD Grade (II): moderate or “pseudo-normal” DD demonstrate abnormal relaxation with increased LV end-diastolic filling pressure and increase the left atrial pressure so (E velocity is greater than A and E/A ratio of 1–2).
DD Grade (III): severe DD demonstrates a further progressive decline in LV compliance (significantly increased LV stiffness) with restrictive filling and atrial contraction is inadequate to force blood into the non-compliant LV (E velocity much higher than A and E/A ratio more than 2). We can differentiate normal diastolic function from DD grade II by using either Valsalva maneuver or by using tissue Doppler imaging, which measures E`wave, which represents the movement of mitral valve annulus if E`< 8 cm means impaired relaxation (DD grade II).
Right ventricle (RV) function: (Figure 3)

RV function was assessed by measuring tricuspid annular systolic excursion (TAPSE) measured in the apical four-chamber image using M-mode. The precursor should be as possible as be aligned along the free wall of RV and perpendicular to the lateral tricuspid annulus, so the precursor becomes parallel to the motion of the tricuspid valve annulus. The tricuspid annulus maximum excursion indicates the distance moved by the leading edge of the tricuspid annulus to the apex measured and expressed in centimeters (from the end of diastole to the end of systole). Normal TAPSE was ≥ 1.6 cm, and impaired TAPSE was < 1.6 cm.
Inferior vena cava (IVC) diameter
IVC was measured using the M-mode imaging in the subcostal view window, and the M-mode precursor is placed roughly 1.0 to 2.0 cm away from the right atrium through the IVC (Figure 4).

IVC diameter was classified as normal (1.5–2.5 cm), collapsed if diameter < 1.5 cm and dilated IVC > 2.5 cm.
SICM patients were defined as a group of the surviving patients who had EF less than 50% and decrease ≥ 10% of the EF compared to the baseline, which recovered within 1–2 weeks. If the baseline measurement appeared uncertain, we assessed EF on admission and defined baseline EF. The concept of recovery based on EF improvement to baseline or > 10% relative to the EF’s initial admission evaluation.3,5
Statistical analysis
Statistical analysis of data was performed by SPSS v26 (IBM©, Chicago, IL, USA). The Kolmogorov–Smirnov test checked the normality of data, and all variables were normally distributed. Quantitative variables were presented as mean and standard deviation (SD) and were compared using the paired t-test. Qualitative variables were presented as frequency and percentage (%) and were analyzed using the chi-square test. Kaplan–Meier survival analysis was used to compare survival between groups. Logistic regression analysis was used to show the independent predictors. The level of significance was adopted at p < 0.05.
Results
Patients' age ranged from 18 to 50 years with a mean value being 38.29 ± 8.71 y. There were 48 (48%) males and 52 (52%) females.
EF was significantly decreased on the 3rd day compared to 1st day (55.64 ± 6.23% vs. 58.24 ± 7.58% respectively (p ˂ 0.001*) then increased again to 60.13 ± 5.57% (p = 0.275). Impaired EF was found in 20 (20%) patients on the 1st day, and only 4 (7%) from 55 surviving patients on the 7th day (significantly decreased compared with 1st day, p = 0.036) (Table 1).

SICM was found in 17 patients from 55 survived patients (Table 2).

Data are presented as mean ± SD or frequency (percentage). n= number of patients, EF: ejection fraction, TAPSE: tricuspid annular systolic excursion, IVC: inferior vena cava, DD: Diastolic dysfunction, HR: heart rate, bpm: beats per minute, MAP: mean arterial blood pressure, CVP: central venous pressure. P1: Comparison between 1st and 3rd day, P2: Comparison between 1st and 7th day *indicates a significant difference as p < 0.05.
Forty-five patients (45%) passed away on the 7th day; 22 patients (22%) died by the 3rd day, and 23 patients (23%) died between the 4th to 7th day.
The comparison between the surviving group and mortality groups on the 7th day showed no significant difference in EF, TAPSE, IVC, and CVP on 1st day. DD on 1st day was significantly higher in the mortality group than in the surviving group (47.7% vs 20%, P = 0.004). HR was significantly higher, and MAP was significantly lower in the mortality group compared to the surviving group (Table 3).

Concerning DD grading, we found that all patients (100%) with DD grade III died, shown the worst prognosis. The mortality percentage related to DD grade I counted as 53.2%, and the least mortality was 32.4%, related to grade II (Table 3).
Regarding patients with impaired systolic function (EF < 50%), there was no significant difference in the mortality group compared with the surviving group (25% vs. 13%, respectively, p = 0.321), indicating that systolic function cannot be used as a predictor of mortality in septic patients (Table 2).
Kaplan–Meier survival analysis
The hazard ratio of mortality was significantly higher 3.06 times (95% CI: 1.26–7.41) in patients with DD compared with patients with normal diastolic function. The hazard ratio of mortality was insignificantly higher [1.59 times (95% CI: 0.76–3.32)] in patients with low EF compared with patients with normal EF. The hazard ratio of mortality was insignificantly lower [0.86 times (95% CI: 0.42–1.77)] in patients with reduced TAPSE compared to patients with normal TAPSE. The hazard ratio of mortality was insignificantly higher [1.38 times (95% CI: 0.71–2.68)] in patients with collapsed IVC compared to patients with normal IVC. The hazard ratio of mortality was significantly higher [3.72 times (95% CI: 1.99–6.94)] in patients with HR > 110 bpm compared to patients with HR ≤ 110 bpm (Figure 5e). The hazard ratio of mortality was significantly higher [9.24 times (95%CI: 3.86–22.11)] in patients with MAP < 65 mmHg compared to patients with MAP ≥ 65 mmHg.
Multiple Logistic Regression Models
In our study, the most independent variables that affect mortality were DD, tachycardia (HR > 110 bpm), and hypotension (MAP < 65 mmHg), which significantly affected the duration of survival negatively (p = 0.0497*, < 0.001, and < 0.001, respectively) (Table 4).

Discussion
Sepsis can lead to reduced LV systolic function, hyperdynamic LV function, and DD. Recognition of these types of MD with echocardiography could result in better outcomes.6 MD, caused by sepsis, is a complicated process because of many factors, including host response to infection, dynamic cardiovascular system (CVS) adaptation to the disease, and resuscitation effects. This entity’s pathophysiology is multifactorial; and extracellular, cellular and systemic mechanisms have been suggested, such as coronary blood flow maldistribution, injury of the myocardium, complementary (C5a) contractile myocyte failure, neutrophil activation by cytokines (TNF, IL-1β, IL-6), Ca manipulation dysregulation, and dysfunction of mitochondria that cause cellular hypoxia.7
Our results revealed that LV systolic function (LVEF), RV function (TAPSE), IVC diameter, and CVP have no role in predicting mortality.
In agreement with our study, Rolando et al.8 studied 53 septic patients, 26% of them had SD, and 23% of them had both SD and DD. They have shown that SD was not a predictor of mortality and uncorrelated with an increased death rate, as compared to DD, which was 84% of patients.
Moreover, Ng et al.9 reported the finding of a crucial study on SIMD comparing septic shock patients (41.9% mortality) with a control group of patients with sepsis (0% mortality), using speckle-tracking echocardiography to identify MD in septic patients. They assessed SD using global longitudinal strain (GLS) and found a higher degree of MD in patients with the septic shock group compared to the control group. On the other side, LVEF was similar in both groups (59% in the study group versus 61% in the control group, p = 0.169).
This finding was in agreement with a meta-analysis recorded by Sanfilippo10 on 581 septic patients. They reported that 29.6% of them had SD, and, there is no correlation between SD and mortality, while 48% of them had DD indicates a significant correlation between DD and mortality.
In contrast to our study, Patil et al. found that the mean LVEF was 35.70% and more than 70% of septic patients had SD, and the mortality rate was high in patients with impaired EF (< 50%) more than patients with normal EF (15% vs. 34%, respectively).11
Another study by Vieillard-Baron and colleagues on 67 patients free from previous cardiac disease and survived for > 48 h stated that global LV hypokinesia (EF < 45%) was found in 60% of the septic patients.12
The left atrium (LA) size is used as a diagnostic criterion for DD. However, due to adaptation to chronically high LV filling pressures, LA enlargement developed slowly. In sepsis and septic shock, LA enlargement does not appear quickly, and diastolic abnormalities are commonly acute. So, in this particular condition, LA size is unlikely to be used as an accurate indicator of diastolic filling abnormalities. Therefore, we chose to use the Doppler evaluation of LV function and filling in a similar situation.12
In our study, the prevalence of DD was 90%, and it was a strong independent predictor of mortality in patients with sepsis and septic shock.
Our results agreed with Sturgess et al.,13 in a retrospective study of 94 general ICU patients who underwent TTE, demonstrated that DD occurred in 67%.
Also, Brown et al.,14 in one of the largest prospective studies assessing 78 septic patients admitted to ICU and underwent TTE within 6 h of admission, after 18 to 32 h for evaluating LVDD, they observed that 36.5% of patients had DD on the 1st ECHO, while 61.8% had DD on at least one ECHO and total mortality was 16.5%. Also, they observed that the largest mortality occurred among patients with grade I DD (37.5%), concluding that DD was a strong independent predictor of mortality in those septic patients.
This agreed with Dantas and Costa,15 as they observed a high alarming occurrence (84%) of DD in septic patients using the E/e’ ratio, which is one of the tissue Doppler imaging (TDI) measurements that used to identify DD, also observed that DD was the only echocardiographic parameter separately correlated with mortality even after age and disease severity adjustment.
The potential influence of DD on the occurrence of mortality of septic patients in ICU can be linked to various factors: First, abnormal relaxation leads to an early decrease of LV filling in addition to an increased proportion of LA contraction to late LV filling and a gradual increase in LV filling pressure. Second, reported tachycardia and hypovolemia with sepsis result in more reduction of LV early loading by shortening the time of diastole and consequently decreasing CO. The significant increase in LV filling pressures presented with DD that may encourage a positive fluid balance, which may exacerbate non-cardiogenic sepsis-related pulmonary edema or may even result in the right cardiac failure. This is indicated by the increased (RVEDA/LVEDA) ratio in ICU dead patients without the association of acute cor-pulmonale.16
From an opposite perspective, there are some studies investigating septic patients in contrast to our results, and these studies revealed that DD has fewer roles in predicting mortality as Bouhemad et al.17 recorded the incidence of LV DD alone 20% in 54 post-operative patients with septic shock using transesophageal echocardiography (TEE).
Also, Gonzalez et al.,18 in their largest study on 540 patients in the ICU with septic shock over 5 years reported that ICU mortality was 35%, and DD was found in 31% of patients. The incidence of DD appeared to be more in the non-survivor group than in the survived group (36% versus 28%, respectively). So, the study concluded that DD could be correlated with ICU mortality, but more studies are needed to declare or prove the prognostic role of DD in septic shock.
Interestingly, in the current study, we found that LV diastolic function was the most significant ECHO parameter. Among patients with normal diastolic function, the mortality rate was 20% (2 patients only from 10 patients presented by normal diastolic function), while the mortality rate was 47.7% among patients with DD, and the worst mortality rate was DD grade III 100% (all patients n=6) followed by DD grade I mortality (53.2%) among its patients (25 patients from 47), then less incidence of mortality was observed in patients with DD grade II (32.4%).
Hemodynamic parameters have an important role in our study. We found some vital data have a pivotal role in predicting mortality in ICU patients, while others do not have any role.
In our study, CVP, as a surrogate for patient volume status, may help in resuscitation with no aid in prognosis or clinical course of septic patients. In contrast to CVP, we found that HR and MAP were strong predictors of mortality in septic patients. Increased HR and decreased MAP in dead patients compared to survived patients reflect that the worst vital data, the worst prognosis, and more increased mortality.
Weng et al.,19 upon following up sepsis and septic shock patients in ICU concluded that HR was significantly lower in the surviving group 103 bpm versus 120 bpm in the non-survived groups. So, HR is a reliable indicator of mortality, while MAP and CVP have no prognostic value in predicting 90-day mortality.
In disagreement with our study Etchecopar-Chevreuil et al.20 in their study, reported that HR and MAP have no value in expecting mortality in ICU septic patients, as MAP was (114 mmHg in the surviving group versus 124 mmHg in the mortality group), also mean HR was (84 bpm in the survived group versus 82 bpm in the mortality group).
The follow-up of 55 survived patients up to the 7th day, we noticed that there was a significant improvement in hemodynamic and echo parameters, compared with the 1st day. When analyzed, these patients were divided into two groups: patients with criteria of SICM (N=17), and others without criteria of SICM (N=38) patients. One of the main finding of this study was precluding a group of patients had SICM criteria (17 patients 31% from survived population and 17% from all study population), in which EF showed initial significant deterioration on the 1st day, then the final significant improvement at the end of the study, as mean LVEF was (48.94 ± 4.71), (52.37 ± 3.76) and (58.59 ± 3.18), on the 1st, 3rd and 7th days respectively (p < 0.001) and patients presented with normal EF ≥ 50% were 3 (18%), 12 (71%) and 17 (100%) patients on the 1st, 3rd and 7th days respectively and patients with impaired EF < 50% were 14 (82%), 5 (29%) patients on the 1st and 3rd days and no patient presented with impaired EF on the 7th day of study.
Other echo parameters showed significant progressive improvement, as mean TAPSE was (1.62 ± 0.20), (1.84 ± 0.25) and (2.07 ± 0.28) cm and mean IVC was (1.52 ± 0.35), (1.79 ± 0.20) and (1.99 ± 0.23) cm on the 1st, 3rd and 7th days, respectively. On the 7th day, the SICM patients’ group was with more improved diastolic function as follows: on the 1st day, there was no patient presented with normal diastolic function, while 17 patients (100%) presented with DD, as (6 patients (35%) with DD grade I and 11 patients (65%) with DD grade II). On the 3rd day, there was only 1 patient (6%) with normal diastolic function, while 16 patients (94%) with DD, as (8 patients (47%) with DD grade I and 8 patients (47%) with DD grade II). On the 7th day, there were 8 patients (47%) with normal diastolic function, while 9 patients (53%) with DD, as (5 patients (29%) with DD grade I and 4 patients (24%) with DD grade II). There was a statistically significant improvement between the 1st and 7th days regarding diastolic function as (p-value 0.003).
Consequently, those patients were diagnosed as SICM patients, facing little reversible impairment of LV systolic function, and they were the groups with a good prognosis.
In agreement with our study Parker and colleagues, after their seminal, works on 20 patients with diagnosed septic shock, as 10 patients suffered from moderate to severe impairment of their EF with values ˂ 40% and 13 of 20 patients survived their episode. Survivors had initial EF with a mean of 32%, and serial scans of the survivor revealed a gradual recovery to normal EF and ventricular volume by 10 days after the beginning of shock. In contrast, non-survivors had normal initial EF and ventricular volumes that did not alter during serial studies.21
The prevalence rate of SICM, recorded in previous research, was 18%-65%22,23 while Sato et al.,5 in their largest study on 210 septic patients demonstrated that SICM patients were 13.8%.
On the other side, Ng et al.9 stated that EF does not appear suitable for assessing MD in septic patients, and no association between EF and mortality. However, caution should be exercised about the analysis of the finding on MD reversibility.
More interestingly, De Geer et al.24 observed no change in systolic function on performing three serial ECHO assessments (on the 1st, 3rd, 4th days, and after ICU discharge) and did not find significant reversibility in GLS. Consequently, MD reversibility questions remain unanswered and need further studies to be explained.
The reliability of the reversibility of SICM depends on the optimum timing of the revaluation echocardiographic examination, which needs to be described. Laboratory studies have documented that lipopolysaccharides in sepsis can produce cardiac fibrosis, which may induce long-term influence on myocardial contractility. Sanfilippo et al.4 selected the time of weaning off vasopressors as an early recovery measurable parameter, during ICU stay of patients. However, to fully understand the extent of myocardial impairment and recovery, follow-up using TTE assessment may be more effectively performed until patients’ full recovery and discharge from ICU.
Although all previous studies that have reported MD in septic shock, most of them could not justify why survivors displayed more marked MD. Levy et al. showed myocardial hibernation in sepsis using positron emission tomography (PET), magnetic resonance imaging (MRI), and single-photon emission tomography. Myocardial hibernation (is an adaptive response to ischemia and hypoxia) is the strongest method to maintain cardiac myocytes by down-regulation of energy requirements and oxygen consumption. It is an adaptive reaction to preserve myocardial viability to prevent the activation of the cell-death pathway and to help full recovery in the future. Also, postmortem examination of septic patients demonstrated a histologic absence of injury and little cell death, although severe organ dysfunction, but more serial study is needed to confirm this hypothesis.25
Septic patients always presented with tachycardia, which worsens LV filling time (decrease diastolic time), and both increased HR and DD are strong predictors of mortality, so many studies were needed to evaluate the benefits of using β-blocker in septic patients for increasing diastolic time, improve LV filling and cardiac performance and its effect on mortality.
Limitations
This study is limited by relatively small sample size, being a single-center study, and a short duration of follow-up (7 days). Also, we did not evaluate the efficacy of therapeutic changes that directly resulted from the initial TTE.
Sepsis-induced myocardial dysfunction; SICM–sepsis-induced cardiomyopathy; TTE– transthoracic echocardiogram
Conclusions
Identification of sepsis-induced myocardial dysfunction using transthoracic echocardiogram in patients with sepsis or septic shock is essential and helpful in the prediction of mortality. Diastolic dysfunction grade III had the highest mortality followed by grade I, grade II), HR >110 bpm, and MAP < 65 mmHg are independent predictors of mortality in those patients. Patients with sepsis-induced cardiomyopathy (little reversible impairment of LV systolic function) had a good prognosis.
Authors’ contributions
All authors contributed to the paper.
Conflict of interest
None declared by the authors.
References
- Lv X, Wang H. Pathophysiology of sepsis-induced myocardial dysfunction. Mil Med Res. 2016;3:30-8. [PubMed] DOI: 1186/s40779-016-0099-9
- Drosatos K, Lymperopoulos A, Kennel PJ, Pollak N, Schulze PC, Goldberg IJ. Pathophysiology of sepsis-related cardiac dysfunction: driven by inflammation, energy mismanagement, or both? Curr Heart Fail Rep. 2015;12:130-40. [PubMed] DOI: 1007/s11897-014-0247-z
- Sato R, Nasu M. A review of sepsis-induced cardiomyopathy. J Intensive Care. 2015;3:48-57. [PubMed] DOI: 10.1186/s40560-015-0112-5
- Hochstadt A, Meroz Y, Landesberg G. Myocardial dysfunction in severe sepsis and septic shock: more questions than answers? J Vasc Surg. 2011;25:526-35. [PubMed] DOI: 1053/j.jvca.2010.11.026
- Aneman A, Vieillard-Baron A. Cardiac dysfunction in sepsis. Intensive Care Med. 2016;42:2073-6. [PubMed] DOI: 1007/s00134-016-4503-4
- Sato R, Kuriyama A, Takada T, Nasu M, Luthe SKe. Prevalence and risk factors of sepsis-induced cardiomyopathy: a retrospective cohort study. Medicine. 2016;95:5031-39. [PubMed] DOI: 1097/MD.0000000000005031
- Sanfilippo F, Santonocito C, Panarello G, Arcadipane A. The role of speckle tracking echocardiography for prognostication in patients with severe sepsis or septic shock. Crit Care Med. 2016;20:284. DOI: /10.1186/s13054-016-1451-x
- Rolando G, Espinoza EDV, Avid E, Welsh S, Pozo JD, Vazquez AR, et al. Prognostic value of ventricular diastolic dysfunction in patients with severe sepsis and septic shock. Rev Bras Ter Intensiva. 2015;27:333-9. [PubMed] DOI: 5935/0103-507X.20150057
- Ng PY, Sin WC, Ng AK-Y, Chan WM. Speckle tracking echocardiography in patients with septic shock: a case control study (SPECKSS). Crit Care. 2016;20:145-53. [PubMed] DOI: 1186/s13054-016-1327-0
- Sanfilippo F, Corredor C, Fletcher N, Landesberg G, Benedetto U, Foex P, et al. Diastolic dysfunction and mortality in septic patients: a systematic review and meta-analysis. Intensive Care Med. 2015;41:1004-13. [PubMed] DOI: 1007/s00134-015-3748-7
- Patil VC, Patil HV, Rajput A, Rao SS, Shetye JN. Relation of echocardiographic parameters to outcome of patients with severe sepsis and septic shock. J Cardiovasc Dis Res. 2017;8:6-15. [FreeFullText]
- Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin FJCcm. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. 2008;36:1701-6. [PubMed] DOI: 1097/CCM.0b013e318174db05
- Sturgess DJ, Marwick TH, Joyce CJ, Jones M, Venkatesh B. Tissue Doppler in critical illness: a retrospective cohort study. Crit Care Med. 2007;11:R97. [PubMed] DOI: 1186/cc6114
- Brown SM, Pittman JE, Hirshberg EL, Jones JP, Lanspa MJ, Kuttler KG, et al. Diastolic dysfunction and mortality in early severe sepsis and septic shock: a prospective, observational echocardiography study. Crit Ultrasound J. 2012;4:8-16. [PubMed] DOI: 1186/2036-7902-4-8
- Dantas VCdS, Costa ELV. A look at the diastolic function in severe sepsis and septic shock. Rev Bras Ter Intensiva. 2015;27:307-8. [PubMed] DOI: 5935/0103-507X.20150052
- Sanfilippo F, Scolletta S, Morelli A, Vieillard-Baron A. Practical approach to diastolic dysfunction in light of the new guidelines and clinical applications in the operating room and in the intensive care. Ann Intensive Care. 2018;8:100. [PubMed] DOI: 1186/s13613-018-0447-x
- Bouhemad B, Nicolas-Robin A, Arbelot C, Arthaud M, Féger F, Rouby J-J. Isolated and reversible impairment of ventricular relaxation in patients with septic shock. Crit Care Med. 2008;36:766-74. [PubMed] DOI: 1097/CCM.0B013E31816596BC
- Gonzalez C, Begot E, Dalmay F, Pichon N, François B, Fedou AL, et al. Prognostic impact of left ventricular diastolic function in patients with septic shock. Ann Intensive Care. 2016;6:36. [PubMed] DOI: 1186/s13613-016-0136-6
- Weng L, Liu YT, Du B, Zhou JF, Guo XX, Peng JM, et al. The prognostic value of left ventricular systolic function measured by tissue Doppler imaging in septic shock. Crit Care Med. 2012;16:R71. [PubMed] DOI: 1186/cc11328
- Etchecopar-Chevreuil C, François B, Clavel M, Pichon N, Gastinne H, Vignon P. Cardiac morphological and functional changes during early septic shock: a transesophageal echocardiographic study. Intensive Care Med. 2008;34:250-6. [PubMed] DOI: 1007/s00134-007-0929-z
- Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100:483-90. [PubMed] DOI: 7326/0003-4819-100-4-483
- Jeong HS, Lee TH, Bang CH, Kim JH, Hong SJ. Risk factors and outcomes of sepsis-induced myocardial dysfunction and stress-induced cardiomyopathy in sepsis or septic shock: A comparative retrospective study. Medicine. 2018;97:e0263-e. [PubMed] DOI: 1097/MD.0000000000010263
- Narváez I, Canabal A, Martín C, Sánchez M, Moron A, Alcalá J, et al. Incidence and evolution of sepsis-induced cardiomyopathy in a cohort of patients with sepsis and septic shock. Med Intensiva. 2018;42:283-91. [PubMed] DOI: 1016/j.medin.2017.08.008
- De Geer L, Engvall J, Oscarsson A. Strain echocardiography in septic shock–a comparison with systolic and diastolic function parameters, cardiac biomarkers and outcome. Crit Care Med. 2015;19:122-7. [PubMed] DOI: 10.1186/s13054-015-0857-1
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138-50. [PubMed] DOI: 10.1056/NEJMra021333