Arjuna Bhagavatha1, Deepa Kattishettar2, Aruna Tegginamatha3
1Junior Resident; 2Assistant Professor; 3Associate Professor
Department of Anesthesiology, Mysore Medical College & Research Institute, South Western Railway Office, Near Mysore Railway Station, Irwin Road, Medar Block, Yadavagiri, Mysuru, Karnataka 570001, (India)
Correspondence: Dr Deepa Kattishettar, #1613, 6th Cross, 6th Main, Vijayanagar 2nd Stage, Mysuru 570017, Karnataka, (India); Phone: 09611104343; E-mail: deepapalax@gmail.com
ABSTRACT
Background and objectives: Intravenous (IV) dexmedetomidine is used as an adjuvant to general anesthesia due to its excellent analgesic and sedation properties. These properties may be useful to prolong the duration of sensory and motor block with spinal anesthesia. Hence, this study was designed to assess the effects of IV dexmedetomidine on the onset, duration and the hemodynamic characteristics of subarachnoid block (SAB) with hyperbaric bupivacaine 0.5% for elective inguinal hernia repair in adult male patients.
Methodology: This prospective randomized double blind study was conducted in 60 adult male patients belonging to American Society of Anesthesiologists (ASA) class I and II undergoing elective inguinal hernia repair. The enrolled patients were divided into 2 groups (n=30) to receive either 0.5 µg/kg dexmedetomidine intravenous bolus over 10 min (Group D) or saline infusion (Group C) prior to subarachnoid block with 0.5% hyperbaric bupivacaine 12.5 mg. Parameters assessed were the time of onset, highest level of sensory block, time to two segment sensory regression, total duration of analgesia, onset and duration of motor block, in addition to hemodynamic parameters at various intervals.
Results: Faster onset time of sensory block (1.29 ± 0.26 min vs. 3.38 ± 0.62 min), prolonged two segment sensory regression time (105.47 ± 8.71min vs. 71.3 ± 9.4 min) and prolonged duration of sensory block (249.5 ± 36.32 min vs. 154.17 ± 13.46 min) were observed in Group D compared to Group C. Similarly, rapid onset (2.92 ± 0.73 min vs. 6.52 ± 0.91 min) and prolonged duration of motor block (192 ± 28.44 min vs135.43 ± 12.64 min) were noted in Group D. Mean sedation score in Group D was 3.83 ± 0.379 vs. 1.83 ± 0.379 in Group C. Time of request for rescue analgesia was significantly prolonged in Group D compared to Group C. Hemodynamic parameters and incidence of side effects were similar in both the groups.
Conclusion: Premedication with single dose of intravenous dexmedetomidine 0.5 µg/kg prior to subarachnoid blockade with 0.5% hyperbaric bupivacaine hastens the onset and increases duration of sensory and motor block, with maintenance of stable hemodynamics and arousable sedation in infra umbilical surgeries in adult male patients.
Key words: Intravenous; Dexmedetomidine; Bupivacaine; Spinal anesthesia
Citation: Bhagavatha A, Kattishettar D, Tegginamatha A. A prospective randomized controlled double blind study of the effects of intravenous dexmedetomidine on subarachnoid block with hyperbaric bupivacaine for elective inguinal hernia repair in adult male patients. Anaesth Pain & Intensive Care 2017;21(2):134-140
Received: 14 Jun 2016, Reviewed: 26 Jun 2016, Corrected: 30 Dec 2016, Accepted: 09 Feb 2017
INTRODUCTION
Spinal anesthesia (SA) is a commonly used regional anesthesia technique in lower abdominal surgeries as it is economical and easy to perform. The intrathecal local anesthetic 0.5% hyperbaric bupivacaine with dextrose is appropriate for surgeries lasting for 2 to 2.5 hours.1 Intrathecal hyperbaric bupivacaine alone is not sufficient to produce postoperative analgesia and hence some adjuvant may have to be added along with local anesthetic. Many adjuvants have been tried to prolong the duration of analgesia and to enhance the spinal anesthetic efficacy. Adjuvants used during SA reduce the dose of local anesthetic agents and their side effects.2,3 Many adjuvants such as opioids (fentanyl), ketamine, neostigmine and alpha-2 agonists like clonidine and dexmedetomidine prolong bupivacaine SA.4,5 One of the drawbacks of using spinal adjuvants when mixed with local anesthetics is the change in pH and baricity of the drug. Drugs have also been used through intravenous routes to enhance the quality of spinal block and prolong the duration of postoperative analgesia.
Dexmedetomidine is a highly selective alpha-2 adrenoceptor agonist, with α2/α1 ratio eight to ten times higher than that of clonidine is an attractive option as an adjuvant during SA.6 Although systemically administered clonidine has been used before bupivacaine SA, not many studies have been done using dexmedetomidine for the same purpose.
We hypothesized that intravenous bolus of dexmedetomidine would prolong the duration of SAB with 0.5% hyperbaric bupivacaine. The aim of our study was to assess the effects of 0.5 µg/ kg of IV dexmedetomidine on the onset and duration of sensory and motor blocks and the effects on hemodynamic characteristics, sedation and adverse effects, when given 10 min before SA with 2.5 ml of 0.5% hyperbaric bupivacaine for elective inguinal hernia repair surgery.
METHODOLOGY
After obtaining approval from institutional ethical committee, male patients aged between 18-55 years, American Society of Anesthesiology (ASA) class I and II, scheduled for elective inguinal hernia repair conducted at our hospital from July 2014 to June 2015, were enrolled for this randomized, controlled double blind clinical study. Written informed consent was taken from every patient. Patients with body mass index (BMI) > 28 kg/m2, patients having any absolute contraindications for SA like raised intracranial pressure, severe hypovolemia, bleeding diathesis, local infection and patients with severe comorbid diseases e.g., diabetes, hypertension, cardiovascular diseases, psychiatric and neurologic diseases, were excluded from the study.
The study population of 60 patients were randomly divided into two groups of 30 each using computer generated randomization table. All the patients received oral alprazolam 0.5 mg and ranitidine hydrochloride 150 mg the night before surgery. Patients were kept nil by mouth overnight. On arrival to the operating room the patients were connected to multiparameter monitor (NIBP, SpO2, and ECG) and the baseline vital parameters were recorded. An IV line with 18G cannula was secured and all patients were preloaded with 10 ml/kg of ringer lactate solution. Group D received IV dexmedetomidine 0.5 µg/kg bolus in 10 ml of normal saline over 10 min before SA. Similarly, Group C received 10 ml of normal saline over 10 min before SA. Study drug was prepared by a senior anesthesiologist not involved in the study. All spinal blockades were performed by the same anesthesiologist who was also an observer. Thus, the patient and the observer were blinded to the study. Ten minutes after the end of the study drug infusion the patients were placed in the flexed lateral position with operative side downwards. The operating table was kept flat and SA was administered in the L3–L4 subarachnoid space through the midline approach with 12.5 mg of hyperbaric bupivacaine. The patients were turned to supine posture immediately and supplemental oxygen was given.
The following parameters were noted in all patients: Onset of sensory and motor blockade, maximum level of sensory and motor blockade attained and the time taken for the same, maximum level of sensory block achieved, time for two segment sensory regression and regression to S1 dermatome, total duration of analgesia, total duration of sensory and motor block were noted. Sensory block was tested using a blunt tipped 27 gauge hypodermic needle in the midaxillary line at every 30 sec for the first 2 min, every minute till the maximum level of block was achieved every 10 min till the end of surgery and thereafter every 30 min until sensory block was resolved. Quality of motor block was assessed by modified Bromage scale7 (0 = no paralysis; 1 = unable to raise extended leg; 2 = unable to flex knee; 3 = unable to flex ankle). Level of sedation was noted using Ramsay Sedation Score8 (1 – Anxious or agitated; 2 – Cooperative and tranquil; 3 - drowsy but responsive to command; 4 – Asleep but responsive to glabellar tap; 5 – Asleep but sluggish response to tactile stimulation; 6 – Asleep and no response). The score was re-assessed every 10 min after drug administration for up to 180 min and every 15 min thereafter till the patient was awake. Excessive sedation was defined as score > 4 out of 6.
Hemodynamic monitoring was done at 1, 3, 7 and 9 min after completion of the study drug and after the subarachnoid block every 1 min for the first 5 min, every 5 min for the next 15 min, every 10 min for the next 30 min and once in 15 min till the end of surgery followed by hourly monitoring in the postoperative period.
Hypotension was defined as more than 20% reduction in the mean arterial pressure below the baseline value and it was treated with fluid boluses and increments of inj mephentermine 3 mg IV. Bradycardia was defined as heart rate less than 50 beats per min and was managed with inj atropine 0.6 mg IV. Total duration of surgery was noted.
Onset of sensory block was defined as the time taken from the completion of intrathecal bupivacaine till the time the patient stopped feeling pin prick at T10 level and onset of motor block when patient attained complete loss of motor power (Bromage 3). Recovery time was defined as the time taken from the maximum level of sensory block attained till the time the sensation regressed by 2 segments.
Patients were monitored during the postoperative period for analgesia and side effects like shivering, sedation, postoperative nausea and vomiting and treated accordingly. Postoperative pain assessment was done using Visual Analogue Scale (0 – 10 cm line). Duration of analgesia was defined as time taken from the completion of sub arachnoid block till the patient complained of pain at the surgical site and inj diclofenac 75 mg intramuscular was given as a rescue analgesic if score was ≥ 4.
Statistical analysis: Data are presented as Mean ± SD or number of patients (percentage) per category. p < 0.05 was considered statistically significant. Sampling was purposive sampling, done using the formula S=z2pq/d2 where z is constant, p is prevalence, q is (1-p) and d is significance level. In this study, considering hospital prevalence of 4% and confidence interval of 95% z was 1.96 and d was 0.05 and applying this formula S= sample size was 60 patients. The data obtained was statistically analyzed using Student’s t test and chi square test using SPSS version 22.
RESULTS
Subarachnoid block was successful in all the patients and all 60 patients completed the study. Demographic parameters with regard to age, height, weight, duration of surgery were comparable between the groups (Table 1).
Table 1: Demographic data
Values are in mean ± SD. NS- Non significant.
Onset of sensory block was significantly faster in Group D (1.29 ± 0.26 min) when compared to Group C (3.38 ± 0.62 min) (p < 0.001). The median maximum level of sensory block was T8 (T6-T10) in Group D compared to T10 (T8-T12) in Group C. The time required for two segment sensory regression was also significantly prolonged in Group D (105.47 ± 8.71 min) when compared to Group C (71.3 ± 9.41 min) (p < 0.001). Mean time for the onset of motor block was significantly shorter in Group D (6.9 ± 0.885 min) in comparison to Group C (13.05 ± 2.08 min) (p < 0.001). The mean duration of motor block was significantly longer in Group D (192 ± 28.44 min) when compared to Group C (135.43 ± 12.64 min) (p < 0.001).The total duration of analgesia was significantly prolonged in Group D (177.67 ± 25.86 min) when compared to Group C (114.2 ± 13.46 min) (Table 2).
The mean Ramsay Sedation Score (RSS) was higher in Group D (3.83 ± 0.329) when compared to Group C (1.83 ± 0.407) which was statistically significant (p < 0.001) (Table 2).
We observed that the trend of mean heart rate in Group D appears to be lower than that of Group C, but there was no statistically significant difference among the groups. The mean heart rates between the groups were maintained between 60 – 85 beats / min indicating the hemodynamic stability in the dexmedetomidine Group at the given dosage. The trend of MAP between the groups showed no significant difference between the groups. But, Group D had lower MAP 3 min after bolus injection of dexmedetomidine up to 20 min after SA when compared to Group C.
The incidence of side effects like hypotension, bradycardia and shivering were not statistically significant between the groups (Table 3)
Table 2: Sensory and Motor blockade characteristics
Values are in mean ± SD. NS- Non significant.
Table 3: Adverse effects
Values are in numbers and percentage. NS- Non significant.
Figure 1: Comparison of intraoperative mean heart rate (HR) in bpm per min between Group D and Group C
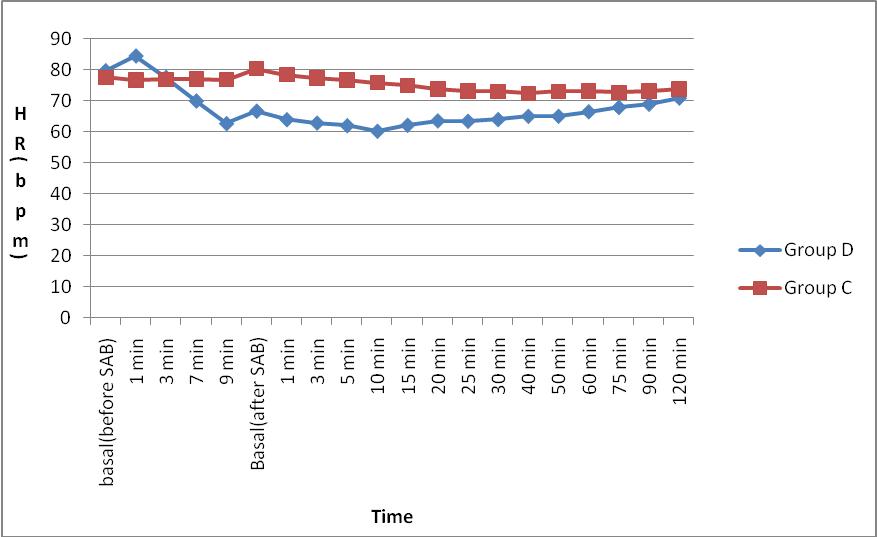
Figure 2: Comparison of intraoperative mean arterial pressure (MAP) in mmHg between Group D and Group C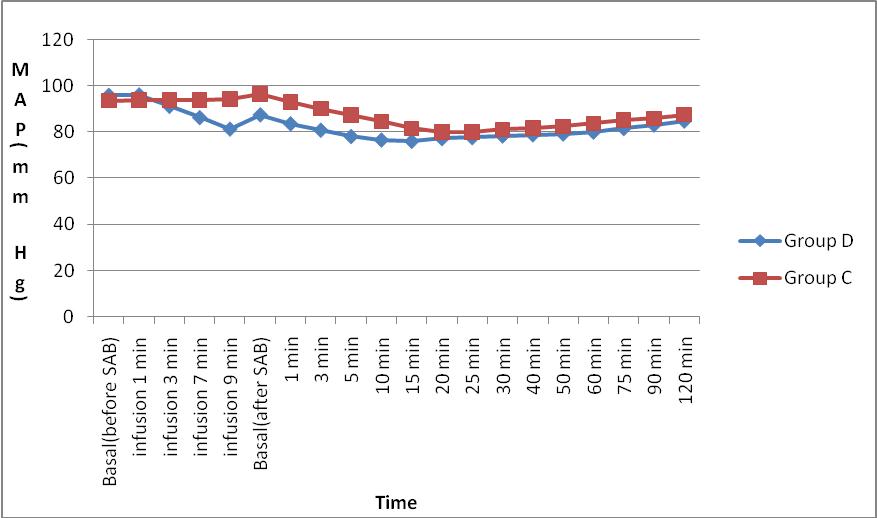
Figure 3: Comparison of Ramsay sedation score (RSS) between Group D and Group C.
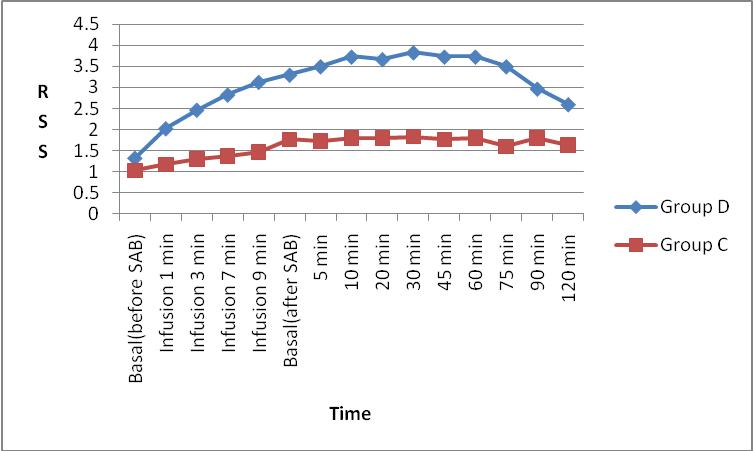
The mean Ramsay Sedation Score (RSS) was higher in Group D when compared to Group C which was statistically significant (p < 0.001).
DISCUSSION
Several techniques and drug regimens have been used from time to time to alleviate the anxiety component and to prolong the postoperative pain relief during regional anesthesia.9,10
Alpha-2 adrenoceptor agonists like clonidine and dexmedetomidine have been used for the same purpose with promising results. The latter is a highly selective α2 adrenoceptor agonist with α2: α1 binding ratio of 1620:1 compared to 220:1 for clonidine.1
Dose range of intravenous dexmedetomidine 0.1 to 10 µg/kg has been commonly used but higher doses have been found to produce significant incidence of bradycardia and hypotension.11,12 An evaluation of the analgesic effects of different doses of intravenous dexmedetomidine (0.25, 0.5, and 1 µg/kg) on ischemic pain in healthy volunteers demonstrated moderate analgesia with a ceiling effect at 0.5 µg/kg.13 Hence, dexmedetomidine 0.5 µg/kg was administered slowly over a period of 10 min in our study.
In our study, injection of dexmedetomidine infusion 0.5 µg/kg bolus prior to subarachnoid block has been found to hasten the onset of sensory block in the study group in comparison to control group (1.29 ± 0.26 min vs. 3.38 ± 0.62 min). The time for peak sensory level was found to be shorter in Group D compared to Group C (4.48 ± 0.549 min vs. 7.1 ± 1.039 min). Similar observations were made by Chandrashekharappa et al.14 who noted significantly shorter onset of sensory block and time for maximum level of sensory block, when dexmedetomidine 0.5 µg/kg was used for IV premedication when compared to the control group. Reddy et al.15 also observed a faster onset of sensory block and time for peak sensory level in dexmedetomidine and clonidine groups. Our study is also comparable to a study by Harsoor et al.16 where dexmedetomidine 0.5 µg/kg was used for IV premedication before subarachnoid block but, maintenance infusion of dexmedetomidine at the rate of 0.5 µg/kg/h was used throughout surgery. They also noted a faster onset of sensory block and shorter time for maximum level of sensory block. The authors suggested that direct analgesic, supraspinal analgesic and/or vasoconstrictive actions of dexmedetomidine may be the mechanism involved.17 The effect seems to be mediated through both presynaptic and postsynaptic α2 receptors.18 In addition, dexmedetomidine infusion may result in increased activation of α2 receptors at the spinal cord resulting in inhibition of nociceptive impulse transmission.16 This mechanism may explain the supraspinal action of intravenous dexmedetomidine in prolongation of subarachnoid block.
The time for two segment regression was prolonged in Group D (105.47 ± 8.71 min) when compared to Group C (71.3 ± 9.41 min). There was a significant prolongation in mean time for two dermatomal regression of sensory blockade in studies by Kaya et al19, Tekin et al.20and Hong et al.21 Similar results were reported by some other researchers.14,15,16,22,23,24 Single dose of intravenous dexmedetomidine was given by Chandrashekharappa et al.14 and Kaya et al.19 All other studies had groups receiving both loading and maintenance doses of intravenous dexmedetomidine.
The mean duration of sensory blockade in our study i.e., time for sensory regression to S1 dermatome is significantly prolonged in Group D (249.5 ± 36.32 min) when compared to Group C (154.17 ± 13.46 min). The mean duration of sensory blockade was significantly prolonged in studies by Al Mustafa et al.,25 and Whizar-Lugo et al.26 In many of the mentioned studies mean duration for sensory regression to S1 dermatome was comparable to our study.14,15,16,23 The total duration of analgesia was significantly prolonged in Group D when compared to the Group C in our study. The time for the first rescue analgesic was also prolonged in other studies.14,21,26
In this present study, the mean time for onset of motor blockade was significantly shorter in Group D (2.92 ± 0.73 min) when compared to Group C (6.52 ± 0.91 min) (p < 0.001). This result is consistent with Reddy et al.15 who noted the time of onset of motor block was reduced by dexmedetomidine (3.64 ± 0.75 min) but not by clonidine (4.21 ± 1.49 min) when compared with placebo (4.57 ± 0.83 min) and Chandrashekharappa et al.14 also noted significant shorter onset of motor blockade in dexmedetomidine group. However, in studies by Kaya et al.19 the mean time for onset of motor blockade was comparable in dexmedetomidine and control groups which was not statistically significant.
In our study, the onset of bradycardia and hypotension was slow and transient. This could be explained by the fact that we used a lower dose of dexmedetomidine infusion at a slow rate. Hemodynamic response following dexmedetomidine infusion depends upon the dose and speed of infusion.16 A sequence of transient increase in blood pressure with reflex bradycardia, followed by hypotension is seen with higher dose and rapid infusion.27,28 Other studies have noted bradycardia as a side effect, with incidences varying from 30–40%. Sometimes requiring treatment with atropine following the use of a bolus dose of 1 µg/kg of dexmedetomidine and maintenance infusion higher than 0.4 µg/kg.22,23 An insignificant decrease in heart rate and blood pressure in patients receiving dexmedetomidine was noted by Harsoor et al.16 and Al Mustafa et al.25
Most of the patients in our study had arousable sedation. The sedation score using modified Ramsay Sedation Score was higher in the Group D compared to Group C. Respiratory depression assessed by fall in saturation was not noted in Group D and SpO2 was maintained well in all of the patients. Harsoor et al.16 and Kaya et al.19 also made similar observations in their studies. Dexmedetomidine induced intra-operative sedation eliminates the need of additional sedatives, thus providing optimal conditions for the surgeon and the patient.29
LIMITATIONS
The limitations of this study were; only male patients were included since the incidence of inguinal hernia is more common in male population. Another limitation was only inguinal hernia surgeries were chosen for this study. However, the results of our study may be applicable to all infraumbilical surgeries in either gender and further studies need to be conducted to assess the same.
CONCLUSION
We conclude that dexmedetomidine 0.5 µg/kg bolus infusion prior to subarachnoid block with hyperbaric bupivacaine quickens the onset of sensory and motor block, prolongs the duration of sensory and motor block and thus postoperative pain relief with minimal changes in hemodynamic profile. It also results in arousable sedation without respiratory depression.
Conflict of interest: Nil declared by the authors
Authors’ contribution:
ABKR: Conduct of study, literature search, statistical analysis
DK & AT: Conduct of study, manuscript editing
REFERENCES
1Junior Resident; 2Assistant Professor; 3Associate Professor
Department of Anesthesiology, Mysore Medical College & Research Institute, South Western Railway Office, Near Mysore Railway Station, Irwin Road, Medar Block, Yadavagiri, Mysuru, Karnataka 570001, (India)
Correspondence: Dr Deepa Kattishettar, #1613, 6th Cross, 6th Main, Vijayanagar 2nd Stage, Mysuru 570017, Karnataka, (India); Phone: 09611104343; E-mail: deepapalax@gmail.com
ABSTRACT
Background and objectives: Intravenous (IV) dexmedetomidine is used as an adjuvant to general anesthesia due to its excellent analgesic and sedation properties. These properties may be useful to prolong the duration of sensory and motor block with spinal anesthesia. Hence, this study was designed to assess the effects of IV dexmedetomidine on the onset, duration and the hemodynamic characteristics of subarachnoid block (SAB) with hyperbaric bupivacaine 0.5% for elective inguinal hernia repair in adult male patients.
Methodology: This prospective randomized double blind study was conducted in 60 adult male patients belonging to American Society of Anesthesiologists (ASA) class I and II undergoing elective inguinal hernia repair. The enrolled patients were divided into 2 groups (n=30) to receive either 0.5 µg/kg dexmedetomidine intravenous bolus over 10 min (Group D) or saline infusion (Group C) prior to subarachnoid block with 0.5% hyperbaric bupivacaine 12.5 mg. Parameters assessed were the time of onset, highest level of sensory block, time to two segment sensory regression, total duration of analgesia, onset and duration of motor block, in addition to hemodynamic parameters at various intervals.
Results: Faster onset time of sensory block (1.29 ± 0.26 min vs. 3.38 ± 0.62 min), prolonged two segment sensory regression time (105.47 ± 8.71min vs. 71.3 ± 9.4 min) and prolonged duration of sensory block (249.5 ± 36.32 min vs. 154.17 ± 13.46 min) were observed in Group D compared to Group C. Similarly, rapid onset (2.92 ± 0.73 min vs. 6.52 ± 0.91 min) and prolonged duration of motor block (192 ± 28.44 min vs135.43 ± 12.64 min) were noted in Group D. Mean sedation score in Group D was 3.83 ± 0.379 vs. 1.83 ± 0.379 in Group C. Time of request for rescue analgesia was significantly prolonged in Group D compared to Group C. Hemodynamic parameters and incidence of side effects were similar in both the groups.
Conclusion: Premedication with single dose of intravenous dexmedetomidine 0.5 µg/kg prior to subarachnoid blockade with 0.5% hyperbaric bupivacaine hastens the onset and increases duration of sensory and motor block, with maintenance of stable hemodynamics and arousable sedation in infra umbilical surgeries in adult male patients.
Key words: Intravenous; Dexmedetomidine; Bupivacaine; Spinal anesthesia
Citation: Bhagavatha A, Kattishettar D, Tegginamatha A. A prospective randomized controlled double blind study of the effects of intravenous dexmedetomidine on subarachnoid block with hyperbaric bupivacaine for elective inguinal hernia repair in adult male patients. Anaesth Pain & Intensive Care 2017;21(2):134-140
Received: 14 Jun 2016, Reviewed: 26 Jun 2016, Corrected: 30 Dec 2016, Accepted: 09 Feb 2017
INTRODUCTION
Spinal anesthesia (SA) is a commonly used regional anesthesia technique in lower abdominal surgeries as it is economical and easy to perform. The intrathecal local anesthetic 0.5% hyperbaric bupivacaine with dextrose is appropriate for surgeries lasting for 2 to 2.5 hours.1 Intrathecal hyperbaric bupivacaine alone is not sufficient to produce postoperative analgesia and hence some adjuvant may have to be added along with local anesthetic. Many adjuvants have been tried to prolong the duration of analgesia and to enhance the spinal anesthetic efficacy. Adjuvants used during SA reduce the dose of local anesthetic agents and their side effects.2,3 Many adjuvants such as opioids (fentanyl), ketamine, neostigmine and alpha-2 agonists like clonidine and dexmedetomidine prolong bupivacaine SA.4,5 One of the drawbacks of using spinal adjuvants when mixed with local anesthetics is the change in pH and baricity of the drug. Drugs have also been used through intravenous routes to enhance the quality of spinal block and prolong the duration of postoperative analgesia.
Dexmedetomidine is a highly selective alpha-2 adrenoceptor agonist, with α2/α1 ratio eight to ten times higher than that of clonidine is an attractive option as an adjuvant during SA.6 Although systemically administered clonidine has been used before bupivacaine SA, not many studies have been done using dexmedetomidine for the same purpose.
We hypothesized that intravenous bolus of dexmedetomidine would prolong the duration of SAB with 0.5% hyperbaric bupivacaine. The aim of our study was to assess the effects of 0.5 µg/ kg of IV dexmedetomidine on the onset and duration of sensory and motor blocks and the effects on hemodynamic characteristics, sedation and adverse effects, when given 10 min before SA with 2.5 ml of 0.5% hyperbaric bupivacaine for elective inguinal hernia repair surgery.
METHODOLOGY
After obtaining approval from institutional ethical committee, male patients aged between 18-55 years, American Society of Anesthesiology (ASA) class I and II, scheduled for elective inguinal hernia repair conducted at our hospital from July 2014 to June 2015, were enrolled for this randomized, controlled double blind clinical study. Written informed consent was taken from every patient. Patients with body mass index (BMI) > 28 kg/m2, patients having any absolute contraindications for SA like raised intracranial pressure, severe hypovolemia, bleeding diathesis, local infection and patients with severe comorbid diseases e.g., diabetes, hypertension, cardiovascular diseases, psychiatric and neurologic diseases, were excluded from the study.
The study population of 60 patients were randomly divided into two groups of 30 each using computer generated randomization table. All the patients received oral alprazolam 0.5 mg and ranitidine hydrochloride 150 mg the night before surgery. Patients were kept nil by mouth overnight. On arrival to the operating room the patients were connected to multiparameter monitor (NIBP, SpO2, and ECG) and the baseline vital parameters were recorded. An IV line with 18G cannula was secured and all patients were preloaded with 10 ml/kg of ringer lactate solution. Group D received IV dexmedetomidine 0.5 µg/kg bolus in 10 ml of normal saline over 10 min before SA. Similarly, Group C received 10 ml of normal saline over 10 min before SA. Study drug was prepared by a senior anesthesiologist not involved in the study. All spinal blockades were performed by the same anesthesiologist who was also an observer. Thus, the patient and the observer were blinded to the study. Ten minutes after the end of the study drug infusion the patients were placed in the flexed lateral position with operative side downwards. The operating table was kept flat and SA was administered in the L3–L4 subarachnoid space through the midline approach with 12.5 mg of hyperbaric bupivacaine. The patients were turned to supine posture immediately and supplemental oxygen was given.
The following parameters were noted in all patients: Onset of sensory and motor blockade, maximum level of sensory and motor blockade attained and the time taken for the same, maximum level of sensory block achieved, time for two segment sensory regression and regression to S1 dermatome, total duration of analgesia, total duration of sensory and motor block were noted. Sensory block was tested using a blunt tipped 27 gauge hypodermic needle in the midaxillary line at every 30 sec for the first 2 min, every minute till the maximum level of block was achieved every 10 min till the end of surgery and thereafter every 30 min until sensory block was resolved. Quality of motor block was assessed by modified Bromage scale7 (0 = no paralysis; 1 = unable to raise extended leg; 2 = unable to flex knee; 3 = unable to flex ankle). Level of sedation was noted using Ramsay Sedation Score8 (1 – Anxious or agitated; 2 – Cooperative and tranquil; 3 - drowsy but responsive to command; 4 – Asleep but responsive to glabellar tap; 5 – Asleep but sluggish response to tactile stimulation; 6 – Asleep and no response). The score was re-assessed every 10 min after drug administration for up to 180 min and every 15 min thereafter till the patient was awake. Excessive sedation was defined as score > 4 out of 6.
Hemodynamic monitoring was done at 1, 3, 7 and 9 min after completion of the study drug and after the subarachnoid block every 1 min for the first 5 min, every 5 min for the next 15 min, every 10 min for the next 30 min and once in 15 min till the end of surgery followed by hourly monitoring in the postoperative period.
Hypotension was defined as more than 20% reduction in the mean arterial pressure below the baseline value and it was treated with fluid boluses and increments of inj mephentermine 3 mg IV. Bradycardia was defined as heart rate less than 50 beats per min and was managed with inj atropine 0.6 mg IV. Total duration of surgery was noted.
Onset of sensory block was defined as the time taken from the completion of intrathecal bupivacaine till the time the patient stopped feeling pin prick at T10 level and onset of motor block when patient attained complete loss of motor power (Bromage 3). Recovery time was defined as the time taken from the maximum level of sensory block attained till the time the sensation regressed by 2 segments.
Patients were monitored during the postoperative period for analgesia and side effects like shivering, sedation, postoperative nausea and vomiting and treated accordingly. Postoperative pain assessment was done using Visual Analogue Scale (0 – 10 cm line). Duration of analgesia was defined as time taken from the completion of sub arachnoid block till the patient complained of pain at the surgical site and inj diclofenac 75 mg intramuscular was given as a rescue analgesic if score was ≥ 4.
Statistical analysis: Data are presented as Mean ± SD or number of patients (percentage) per category. p < 0.05 was considered statistically significant. Sampling was purposive sampling, done using the formula S=z2pq/d2 where z is constant, p is prevalence, q is (1-p) and d is significance level. In this study, considering hospital prevalence of 4% and confidence interval of 95% z was 1.96 and d was 0.05 and applying this formula S= sample size was 60 patients. The data obtained was statistically analyzed using Student’s t test and chi square test using SPSS version 22.
RESULTS
Subarachnoid block was successful in all the patients and all 60 patients completed the study. Demographic parameters with regard to age, height, weight, duration of surgery were comparable between the groups (Table 1).
Table 1: Demographic data
| Parameters | Group D | Group C | p value |
| Mean age (y) | 40.60 ± 7.90 | 40.07 ± 6.82 | NS |
| Mean weight (kg) | 65.03 ± 5.83 | 61.80 ± 4.90 | NS |
Onset of sensory block was significantly faster in Group D (1.29 ± 0.26 min) when compared to Group C (3.38 ± 0.62 min) (p < 0.001). The median maximum level of sensory block was T8 (T6-T10) in Group D compared to T10 (T8-T12) in Group C. The time required for two segment sensory regression was also significantly prolonged in Group D (105.47 ± 8.71 min) when compared to Group C (71.3 ± 9.41 min) (p < 0.001). Mean time for the onset of motor block was significantly shorter in Group D (6.9 ± 0.885 min) in comparison to Group C (13.05 ± 2.08 min) (p < 0.001). The mean duration of motor block was significantly longer in Group D (192 ± 28.44 min) when compared to Group C (135.43 ± 12.64 min) (p < 0.001).The total duration of analgesia was significantly prolonged in Group D (177.67 ± 25.86 min) when compared to Group C (114.2 ± 13.46 min) (Table 2).
The mean Ramsay Sedation Score (RSS) was higher in Group D (3.83 ± 0.329) when compared to Group C (1.83 ± 0.407) which was statistically significant (p < 0.001) (Table 2).
We observed that the trend of mean heart rate in Group D appears to be lower than that of Group C, but there was no statistically significant difference among the groups. The mean heart rates between the groups were maintained between 60 – 85 beats / min indicating the hemodynamic stability in the dexmedetomidine Group at the given dosage. The trend of MAP between the groups showed no significant difference between the groups. But, Group D had lower MAP 3 min after bolus injection of dexmedetomidine up to 20 min after SA when compared to Group C.
The incidence of side effects like hypotension, bradycardia and shivering were not statistically significant between the groups (Table 3)
Table 2: Sensory and Motor blockade characteristics
| Parameters | Group D | Group C | p value |
| Mean duration of surgery (min) | 82 ± 9.05 | 73 ± 14.12 | NS |
| Mean time of onset of sensory analgesia at T10 (min) | 1.29 ± 0.26 | 3.38 ± 0.62 | < 0.001 |
| Mean time to achieve highest level of sensory block (min) | 4.48 ± 0.55 | 7.10 ± 1.03 | < 0.001 |
| Median maximum level of sensory blockade | T8(T6-T10) | T10(T8-T12) | NS |
| Mean time for two segment sensory regression (min) | 105.47 ± 8.71 | 71.3 ± 9.41 | < 0.001 |
| Mean time for sensory regression to S1(min) | 249.50 ± 36.32 | 154.7 ± 13.46 | < 0.001 |
| Mean time of onset of Grade I motor block (min) | 2.92 ± 0.73 | 6.52 ± 0.91 | < 0.001 |
| Mean time of onset of Grade IV motor block (min) | 6.9 ± 0.885 | 13.05 ± 2.08 | < 0.001 |
| Mean duration of motor block (min) | 192 ± 28.44 | 135.43 ± 12.64 | < 0.001 |
| Mean total duration of analgesia (min) | 177.67 ± 25.86 | 114.2 ± 13.46 | < 0.001 |
| Sedation score (by Modified Ramsay Sedation Score) | 3.83 ± .329 | 1.83 ± .407 | < 0.001 |
Table 3: Adverse effects
| Parameters | Group D | Group C | P value |
| Hypotension | 4 (11.33) | 0 (0) | NS |
| Bradycardia | 2 (6.66) | 0 (0) | NS |
| Shivering | 0 | 3 (10) | NS |
Figure 1: Comparison of intraoperative mean heart rate (HR) in bpm per min between Group D and Group C

Figure 2: Comparison of intraoperative mean arterial pressure (MAP) in mmHg between Group D and Group C

Figure 3: Comparison of Ramsay sedation score (RSS) between Group D and Group C.

The mean Ramsay Sedation Score (RSS) was higher in Group D when compared to Group C which was statistically significant (p < 0.001).
DISCUSSION
Several techniques and drug regimens have been used from time to time to alleviate the anxiety component and to prolong the postoperative pain relief during regional anesthesia.9,10
Alpha-2 adrenoceptor agonists like clonidine and dexmedetomidine have been used for the same purpose with promising results. The latter is a highly selective α2 adrenoceptor agonist with α2: α1 binding ratio of 1620:1 compared to 220:1 for clonidine.1
Dose range of intravenous dexmedetomidine 0.1 to 10 µg/kg has been commonly used but higher doses have been found to produce significant incidence of bradycardia and hypotension.11,12 An evaluation of the analgesic effects of different doses of intravenous dexmedetomidine (0.25, 0.5, and 1 µg/kg) on ischemic pain in healthy volunteers demonstrated moderate analgesia with a ceiling effect at 0.5 µg/kg.13 Hence, dexmedetomidine 0.5 µg/kg was administered slowly over a period of 10 min in our study.
In our study, injection of dexmedetomidine infusion 0.5 µg/kg bolus prior to subarachnoid block has been found to hasten the onset of sensory block in the study group in comparison to control group (1.29 ± 0.26 min vs. 3.38 ± 0.62 min). The time for peak sensory level was found to be shorter in Group D compared to Group C (4.48 ± 0.549 min vs. 7.1 ± 1.039 min). Similar observations were made by Chandrashekharappa et al.14 who noted significantly shorter onset of sensory block and time for maximum level of sensory block, when dexmedetomidine 0.5 µg/kg was used for IV premedication when compared to the control group. Reddy et al.15 also observed a faster onset of sensory block and time for peak sensory level in dexmedetomidine and clonidine groups. Our study is also comparable to a study by Harsoor et al.16 where dexmedetomidine 0.5 µg/kg was used for IV premedication before subarachnoid block but, maintenance infusion of dexmedetomidine at the rate of 0.5 µg/kg/h was used throughout surgery. They also noted a faster onset of sensory block and shorter time for maximum level of sensory block. The authors suggested that direct analgesic, supraspinal analgesic and/or vasoconstrictive actions of dexmedetomidine may be the mechanism involved.17 The effect seems to be mediated through both presynaptic and postsynaptic α2 receptors.18 In addition, dexmedetomidine infusion may result in increased activation of α2 receptors at the spinal cord resulting in inhibition of nociceptive impulse transmission.16 This mechanism may explain the supraspinal action of intravenous dexmedetomidine in prolongation of subarachnoid block.
The time for two segment regression was prolonged in Group D (105.47 ± 8.71 min) when compared to Group C (71.3 ± 9.41 min). There was a significant prolongation in mean time for two dermatomal regression of sensory blockade in studies by Kaya et al19, Tekin et al.20and Hong et al.21 Similar results were reported by some other researchers.14,15,16,22,23,24 Single dose of intravenous dexmedetomidine was given by Chandrashekharappa et al.14 and Kaya et al.19 All other studies had groups receiving both loading and maintenance doses of intravenous dexmedetomidine.
The mean duration of sensory blockade in our study i.e., time for sensory regression to S1 dermatome is significantly prolonged in Group D (249.5 ± 36.32 min) when compared to Group C (154.17 ± 13.46 min). The mean duration of sensory blockade was significantly prolonged in studies by Al Mustafa et al.,25 and Whizar-Lugo et al.26 In many of the mentioned studies mean duration for sensory regression to S1 dermatome was comparable to our study.14,15,16,23 The total duration of analgesia was significantly prolonged in Group D when compared to the Group C in our study. The time for the first rescue analgesic was also prolonged in other studies.14,21,26
In this present study, the mean time for onset of motor blockade was significantly shorter in Group D (2.92 ± 0.73 min) when compared to Group C (6.52 ± 0.91 min) (p < 0.001). This result is consistent with Reddy et al.15 who noted the time of onset of motor block was reduced by dexmedetomidine (3.64 ± 0.75 min) but not by clonidine (4.21 ± 1.49 min) when compared with placebo (4.57 ± 0.83 min) and Chandrashekharappa et al.14 also noted significant shorter onset of motor blockade in dexmedetomidine group. However, in studies by Kaya et al.19 the mean time for onset of motor blockade was comparable in dexmedetomidine and control groups which was not statistically significant.
In our study, the onset of bradycardia and hypotension was slow and transient. This could be explained by the fact that we used a lower dose of dexmedetomidine infusion at a slow rate. Hemodynamic response following dexmedetomidine infusion depends upon the dose and speed of infusion.16 A sequence of transient increase in blood pressure with reflex bradycardia, followed by hypotension is seen with higher dose and rapid infusion.27,28 Other studies have noted bradycardia as a side effect, with incidences varying from 30–40%. Sometimes requiring treatment with atropine following the use of a bolus dose of 1 µg/kg of dexmedetomidine and maintenance infusion higher than 0.4 µg/kg.22,23 An insignificant decrease in heart rate and blood pressure in patients receiving dexmedetomidine was noted by Harsoor et al.16 and Al Mustafa et al.25
Most of the patients in our study had arousable sedation. The sedation score using modified Ramsay Sedation Score was higher in the Group D compared to Group C. Respiratory depression assessed by fall in saturation was not noted in Group D and SpO2 was maintained well in all of the patients. Harsoor et al.16 and Kaya et al.19 also made similar observations in their studies. Dexmedetomidine induced intra-operative sedation eliminates the need of additional sedatives, thus providing optimal conditions for the surgeon and the patient.29
LIMITATIONS
The limitations of this study were; only male patients were included since the incidence of inguinal hernia is more common in male population. Another limitation was only inguinal hernia surgeries were chosen for this study. However, the results of our study may be applicable to all infraumbilical surgeries in either gender and further studies need to be conducted to assess the same.
CONCLUSION
We conclude that dexmedetomidine 0.5 µg/kg bolus infusion prior to subarachnoid block with hyperbaric bupivacaine quickens the onset of sensory and motor block, prolongs the duration of sensory and motor block and thus postoperative pain relief with minimal changes in hemodynamic profile. It also results in arousable sedation without respiratory depression.
Conflict of interest: Nil declared by the authors
Authors’ contribution:
ABKR: Conduct of study, literature search, statistical analysis
DK & AT: Conduct of study, manuscript editing
REFERENCES
- Miller RD, Erikson LI, Fleisher LA, Wiener-Kronish GP, Young WI. Spinal epidural and caudal anesthesia. Miller’s Anaesthesia. . Philadelphia PA: Elsevier Churchill Livingstone. 2010;7:1624.
- Chilvers CR, Goodwin A, Vaghadia H, Mitchell GW. Selective spinal anesthesia for outpatient laparoscopy. V: Pharmacoeconomic comparison vs general anesthesia. Can J Anaesth. 2001;48:279–83 [PubMed]
- Liu SS, McDonald SB. Current issues in spinal anesthesia. Anesthesiology. 2001;94:888–906. [PubMed]
- MikkoPitkänen. Spinal (Subarachnoid) blockade. In: Cousin MJ, Bridenbaugh PO, Carr DB, Horlocker TT, editors, Neural Blockade in Clinical Anaesthesia and Management of Pain. 4th ed. Philadelphia: Lippincot Williams and Wilkins; 2009. pp.213-38
- Rhee K, Kang K, Kim J, Jeon Y. Intravenous clonidine prolongs bupivacaine spinal anesthesia. Acta Anaesthesiol Scand. 2003;47:1001–5 [PubMed]
- Venn RM, Bradshaw CJ, Spencer R. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54:1136-142 [PubMed]
- Bromage PR, Burfoot MF, Crowell DE, Pettigrew RT. Quality of epidural blockade. I. influence of physical factors. Br J Anaesth 1964;36:34252. [PubMed]
- Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone–alphadolone. Br Med J 1974;2:656-9. [PubMed]
- Hohener D, Blumenthal S, Borgeat A. Sedation and regional anaesthesia in the adult patient. Br J Anaesth.2008;100:8–16. [PubMed]
- Helgeson LE. Sedation during regional anaesthesia: Inhalational versus intravenous. Curr Opin Anaesthesiol. 2005;18:534–9. [PubMed]
- Feld JM, Hoffman WE, Stechert MM, Hoffman IW, Ananda RC. Fentanyl or dexmedetomidine combined with desflurane for bariatric surgery. J Clin Anesth. 2006;18:24–8 [PubMed]
- Ramsey MA, Saha D, Hebeler RF. Tracheal resection in the morbidly obese patient: The role of dexmedetomidine. J Clin Anesth. 2006;18:452–4. [PubMed]
- Jaakola ML, Salonen M, Lehtinen R, Scheinin H. The analgesic action of dexmedetomidine-a novel α2-adrenoceptor agonist-in healthy volunteers. Pain. 1991;46:281–5. [PubMed]
- Chandrashekharappa K, Ravindra C G, Kumara A B, Kiran M. Intravenous Dexmedetomidine Premedication on spinal Anaesthesia with Hyperbaric Bupivacaine in Patients Undergoing Total Abdominal Hysterectomies. IJHSR. (2015); 5(8): 155- 61. [Online] [Free full text]
- Reddy VS, Shaik NA, Donthu B, Reddy Sannala VK, Jangam V. Intravenous dexmedetomidine versus clonidine for prolongation of bupivacaine spinal anesthesia and analgesia: A randomized double-blind study. J Anaesthesiol Clin Pharmacol. 2013 Jul-Sep; 29(3): 342–7. [PubMed]
- Harsoor SS, Rani DD, Yalamuru B, Sudheesh K, Nethra SS. Effect of supplementation of low dose intravenous dexmedetomidine on characteristics of spinal anaesthesia with hyperbaric bupivacaine. Indian J Anaesth 2013;57:265-9. [PubMed] [Free full text] DOI:4103/0019-5049.115616
- Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 2000; 382–94 [Pubmed].
- Coursin DB, Maccioli GA. Dexmedetomidine. Curr Opin Crit Care2001;7:2216. [PubMed]
- Kaya FN, Yavascaoglu B, Turker G, Yildirim A, Gurbet A, Mogol EB, et al. Intravenous dexmedetomidine, but not midazolam, prolongs bupivacaine spinal anesthesia. Can J Anaes 2010;57:39-45. [PubMed]
- Tekin M, Kati I, Tomak Y, Kisli E. Effect of dexmedetomidine IV on the duration of spinal anesthesia with Prilocaine: a double-blind, prospective study in adult surgical patients. Curr Ther Res Clin Exp. 2007;68:313-24. [PubMed]
- Hong JY, Kim WO, Yoon Y, Choi Y, Kim SH, Kil HK. Effects of intravenous dexmedetomidine on low-dose bupivacaine spinal anaesthesia in elderly patients. Acta Anaesthesiol Scand 2012;56:382-7. [PubMed]
- Elcıcek K, Tekin M, Kati I. The effects of intravenous dexmedetomidine on spinal hyperbaric ropivacaineanesthesia. J Anesth 2010;24:544-8. [PubMed]
- Gupta K, Tiwari V, Gupta PK, Pandey MN, Agarwal S, Arora A. Prolongation of subarachnoid block by intravenous dexmedetomidine for sub umbilical surgical procedures: A prospective control study. Anesth Essays Res. 2014 May-Aug; 8(2): 175–8. [PubMed]
- Kiran Kumar S, Anand kumar C, Murthy SGK. A Comparative Prospective, Randomised, Double Blind Study of the Effect of IV Dexmedetomidine on Subarachnoid Block Versus 0.9 % Normal Saline as Control. Sch J App Med Sci 2014; 2(5A):1517-23. [Full Link]
- Al-Mustafa MM, Badran IZ, Abu Ali HM, Al-Barazangi BA, Massad IM, Al-Ghanem SM. Intravenous dexmedetomidine prolongs bupivacine spinal analgesia. Middle East J Anaesthesiol; 2009. [PubMed]
- Whizar-Lugo V, Gómez-Ramírez IA, Cisneros-Corral R, Martínez-Gallegos N. Intravenous dexmedetomidine vs. intravenous clonidine to prolong bupivacaine spinal anaesthesia. A double blind study. Anestesiaen Mexico 2007;19:143-6. [Free full text]
- Mason KP, Zurakowski D, Zgleszewski S, Prescilla R. Fontaine PJ, Dinardo JA. Incidence and predictors of hypertension during high - dose dexmedetomidine sedation for paediatric MRI. Paediatr Anaesth 2010;20:516 [PubMed]
- Sudheesh K, Harsoor S. Dexmedetomidine in anaesthesia practice: A wonder drug? Indian J Anaesth 2011;55:323-4. [Link]
- Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small – dose dexmedetomidine infusions. Anesth Analg 2000;90:699-705.[PubMed]